Smac mimetics: implications for enhancement of targeted therapies in leukemia - PubMed (original) (raw)
. 2010 Dec;24(12):2100-9.
doi: 10.1038/leu.2010.212. Epub 2010 Sep 16.
A Ray, R Barrett, E Nelson, A L Christie, D Porter, C Straub, L Zawel, J F Daley, S Lazo-Kallanian, R Stone, I Galinsky, D Frank, A L Kung, J D Griffin
Affiliations
- PMID: 20844561
- PMCID: PMC4037865
- DOI: 10.1038/leu.2010.212
Smac mimetics: implications for enhancement of targeted therapies in leukemia
E Weisberg et al. Leukemia. 2010 Dec.
Erratum in
- Leukemia. 2011 Jul;25(7):1221
Abstract
Drug resistance is a growing concern with clinical use of tyrosine kinase inhibitors. Utilizing in vitro models of intrinsic drug resistance and stromal-mediated chemoresistance, as well as functional mouse models of progressive and residual disease, we attempted to develop a potential therapeutic approach designed to suppress leukemia recurrence following treatment with selective kinase inhibitors. The novel IAP inhibitor, LCL161, [corrected] was observed to potentiate the effects of tyrosine kinase inhibition against leukemic disease both in the absence and presence of a stromal-protected [corrected] environment. LCL161 enhanced the proapoptotic effects of nilotinib and PKC412, against leukemic disease in vitro and potentiated the activity of both kinase inhibitors against leukemic disease in vivo. In addition, LCL161 synergized in vivo with nilotinib to reduce leukemia burden significantly below the baseline level suppression exhibited by a moderate-to-high dose of nilotinib. Finally, LCL161 displayed antiproliferative effects against cells characterized by intrinsic resistance to tyrosine kinase inhibitors as a result of expression of point mutations in the protein targets of drug inhibition. These results support the idea of using IAP inhibitors in conjunction with targeted tyrosine kinase inhibition to override drug resistance and suppress or eradicate residual disease.
Conflict of interest statement
Conflict of Interest:
J.D.G., R.S., D.F. and A.L.K. have a financial interest with Novartis Pharma AG. Employees of Novartis include D.P., C.S., and L.Z. (formerly).
Figures
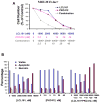
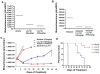
Figure 1. Effects of IAP inhibitor treatment and FLT3 inhibition, alone and combined, on the growth and viability of mutant FLT3-expressing cells in vitro
(A) Approximately 3-day treatment of MOLM13 cells with LCL161, PKC412, or a combination of LCL161+PKC412. Calcusyn-derived combination indices derived from data plotted in (A) are shown in Table 1. Data shown are representative of three independent studies. (B) Induction of apoptosis of MOLM13-luc+ cells by LCL161, PKC412, or a combination of the two agents following treatment for approximately 3 days. Data shown are representative of two independent studies.
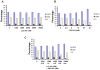
Figure 2. Analysis of cell cycle progression of LCL161−, PKC412−, or combination-treated MOLM13-luc+ cells
(A–C). 48 hour time point.
Figure 3. Effects of SCM on kinase inhibitor-treated leukemia cells in vitro
(A) MOLM13-luc+ cells treated for approximately 3 days by PKC412 in the absence and presence of SCM. Media was collected from HS-5 stromal cells after 1 week from an already-established monolayer. HS-5 stromal cells were originally seeded 12 days prior to removal of conditioned media. Cells were then further cultured in fresh media for 7 days. Conditioned media was then collected for use in this study. (B) Approximately 3-day treatment of Ba/F3-FLT3-ITD cells in the presence and absence of SCM from primary murine stroma. Primary stromal cells were seeded following femur flush from C67BL/6J mice. SCM was collected and pooled from an already-established mouse primary stroma monolayer. Cells were cultured for 16 days in fresh media prior to media pool and collection. (C) Proliferation studies of MOLM13-luc+ cells treated for approximately 3 days by PKC412+/−LCL161 in the presence of HS-5 SCM. Cell counts were obtained via Trypan Blue exclusion. (D) Viability assays corresponding to study shown in C. Data points shown are the percentage of viable cells (as determined by Annexin-V-Fluos Staining) shown as a percent of untreated controls.
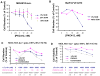
Figure 4. Effects of IAP inhibitor treatment and FLT3 inhibition, alone and combined, on the growth of mutant FLT3-expressing cells in vivo
(A–C) In vivo bioluminescence imaging study. Male NCr nude mice were administered, via tail vein injection, approximately 800,000 Ba/F3-FLT3-ITD-luc+ cells. Baseline imaging and randomization of mice were performed on day 1 post-IV injection of cells. Drug treatments were carried out for a total of 7 days, with final imaging performed on day 8 post-IV injection of cells. Results of Student t-test for statistical analysis of in vivo bioluminescence assay: Vehicle vs PKC412 (p=0.047); Vehicle vs LCL161 (p=0.142); Vehicle vs combination (p=0.028); PKC412 vs LCL161 (p=0.1505); PKC412 vs combination (p=0.024); LCL161 vs combination (p=0.032). (A) Mouse images obtained on day 8 post-IV injection of cells. (B) Plotted bioluminescence values for entire study. (C) Percent spleen weights obtained for mice sacrificed 9 days following the final imaging day. Vehicle mice: 2 viable at time of sacrifice. PKC412-tr mice: 3 viable at time of sacrifice. LCL161-tr mice: 2 viable at time of sacrifice. Combination-treated mice: 4 viable at time of sacrifice.
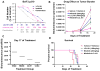
Figure 5. Effects of IAP inhibitor treatment and Abl inhibition, alone and combined, on the growth of BCR-ABL-expressing cells in vitro and in vivo
(A) Approximately 3-day treatment of Ba/F3.p210 cells with LCL161, imatinib, or a combination of LCL161+imatinib. Calcusyn-derived combination indices are shown in Table 1. (B–D) In vivo bioluminescence imaging study. Male NCr nude mice were administered, via tail vein injection, approximately 800,000 Ba/F3-FLT3-ITD-luc+ cells. 32 mice were injected with 32D-p210-LucNeo cells and imaged 3 days later to determine tumor burden. Following randomization, mice were divided into 4 treatment groups (n=8). Treatments were carried out via oral gavage 5X per week for a total of 3 weeks. Mice were imaged every 4–8 days and time-to-sac was recorded. Mice were administered vehicle, nilotinib at 20mg/kg 1X daily, LCL161 at 100mg/kg 1X daily, or a combination of nilotinib+LCL161. (B) Plotted bioluminescence values for entire study. (C) Plotted bioluminescence values on Day 17 of treatment (p=0.0002, ANOVA). (D) Survival curve (p < 0.0001, ANOVA).
Figure 6. Enhancement of in vivo effects of high-moderate doses of nilotinib by LCL161 on leukemia burden in mice
(A–B) In vivo bioluminescence study investigating the effects of leukemia burden by short-term (4-day) treatment of BCR-ABL-harboring mice with high dose (100mg/kg) nilotinib, +/−LCL161 treatment. NCr nude mice (n=6 per treatment group) were injected via tail vein with 800,000 32D.p210-luc+ cells, and treated for a total of 4 days with vehicle, nilotinib (100mg/kg), LCL161 (40mg/kg), LCL161 (100mg/kg), a combination of nilotinib (100mg/kg)+LCL161 (40mg/kg), or a combination of nilotinib (100mg/kg)+LCL161 (100mg/kg). (A) Plotted bioluminescence values showing the effects of LCL161 on leukemia burden, as compared to vehicle control-treated mice, on Day 5 of treatment (p=0.0007, ANOVA). (B) Plotted bioluminescence values showing the effects of nilotinib alone, nilotinib+LCL161 (40mg/kg), or nilotinib+LCL161 (100mg/kg) on leukemia burden on Day 13 of treatment (p=0.002, ANOVA). (C) In vivo bioluminescence study investigating the effects on leukemia burden by long-term (several week) treatment of BCR-ABL-harboring mice with moderate-high dose (75mg/kg) nilotinib, +/−LCL161 treatment. NCr nude mice (n=9 for nilotinib and vehicle; n=10 for LCL161 and combination) were injected via tail vein with 800,000 32D.p210-luc+ cells, and treated for a total of 5 weeks with vehicle, nilotinib (75mg/kg 1X daily), LCL161 (100 mg/kg 1X daily), or a combination of nilotinib+LCL161. (C) Plotted bioluminescence values. ANOVA analysis of BLI comparing all groups at Day 8 of treatment (when all animals still alive) has p value <0.0001. Comparison of nilotinib to combo BLI on day 47 of treatment (last point before significant deaths in nilotinib group) p=0.04. (D) Survival curves. Survival comparison of all groups p<0.0001. Survival comparison of nilotinib and combo p=0.003.
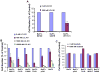
Figure 7. Inhibition of proliferation of drug-resistant cell lines by LCL161
(A) Approximately 2-day LCL161 treatment of N676D-Ba/F3 cells, G697R-Ba/F3 cells, and wt FLT3-Ba/F3 cells. (B) Approximately 2-day LCL161 treatment of parental Ba/F3 cells, Ba/F3.p210, and Ba/F3 cells expressing the imatinib-resistant BCR-ABL mutants M351T, F317L, F486S, and T315I.. (C) Approximately 2-day imatinib treatment of Ba/F3, Ba/F3.p210, and Ba/F3 cells expressing imatinib-resistant BCR-ABL point mutants, shown as a control.
Similar articles
- Using combination therapy to override stromal-mediated chemoresistance in mutant FLT3-positive AML: synergism between FLT3 inhibitors, dasatinib/multi-targeted inhibitors and JAK inhibitors.
Weisberg E, Liu Q, Nelson E, Kung AL, Christie AL, Bronson R, Sattler M, Sanda T, Zhao Z, Hur W, Mitsiades C, Smith R, Daley JF, Stone R, Galinsky I, Griffin JD, Gray N. Weisberg E, et al. Leukemia. 2012 Oct;26(10):2233-44. doi: 10.1038/leu.2012.96. Epub 2012 Apr 3. Leukemia. 2012. PMID: 22469781 Free PMC article. - Potentiation of antileukemic therapies by Smac mimetic, LBW242: effects on mutant FLT3-expressing cells.
Weisberg E, Kung AL, Wright RD, Moreno D, Catley L, Ray A, Zawel L, Tran M, Cools J, Gilliland G, Mitsiades C, McMillin DW, Jiang J, Hall-Meyers E, Griffin JD. Weisberg E, et al. Mol Cancer Ther. 2007 Jul;6(7):1951-61. doi: 10.1158/1535-7163.MCT-06-0810. Mol Cancer Ther. 2007. PMID: 17620426 - Selective Akt inhibitors synergize with tyrosine kinase inhibitors and effectively override stroma-associated cytoprotection of mutant FLT3-positive AML cells.
Weisberg E, Liu Q, Zhang X, Nelson E, Sattler M, Liu F, Nicolais M, Zhang J, Mitsiades C, Smith RW, Stone R, Galinsky I, Nonami A, Griffin JD, Gray N. Weisberg E, et al. PLoS One. 2013;8(2):e56473. doi: 10.1371/journal.pone.0056473. Epub 2013 Feb 21. PLoS One. 2013. PMID: 23437141 Free PMC article. - FLT3 inhibitors in acute myeloid leukemia: Current and future.
Thomas CM, Campbell P. Thomas CM, et al. J Oncol Pharm Pract. 2019 Jan;25(1):163-171. doi: 10.1177/1078155218802620. Epub 2018 Sep 30. J Oncol Pharm Pract. 2019. PMID: 30270754 Review. - Investigational FMS-like tyrosine kinase 3 inhibitors in treatment of acute myeloid leukemia.
Pemmaraju N, Kantarjian H, Andreeff M, Cortes J, Ravandi F. Pemmaraju N, et al. Expert Opin Investig Drugs. 2014 Jul;23(7):943-54. doi: 10.1517/13543784.2014.911839. Epub 2014 Apr 21. Expert Opin Investig Drugs. 2014. PMID: 24749672 Free PMC article. Review.
Cited by
- Using combination therapy to override stromal-mediated chemoresistance in mutant FLT3-positive AML: synergism between FLT3 inhibitors, dasatinib/multi-targeted inhibitors and JAK inhibitors.
Weisberg E, Liu Q, Nelson E, Kung AL, Christie AL, Bronson R, Sattler M, Sanda T, Zhao Z, Hur W, Mitsiades C, Smith R, Daley JF, Stone R, Galinsky I, Griffin JD, Gray N. Weisberg E, et al. Leukemia. 2012 Oct;26(10):2233-44. doi: 10.1038/leu.2012.96. Epub 2012 Apr 3. Leukemia. 2012. PMID: 22469781 Free PMC article. - Therapeutic targeting of necroptosis by Smac mimetic bypasses apoptosis resistance in acute myeloid leukemia cells.
Safferthal C, Rohde K, Fulda S. Safferthal C, et al. Oncogene. 2017 Mar;36(11):1487-1502. doi: 10.1038/onc.2016.310. Epub 2016 Nov 21. Oncogene. 2017. PMID: 27869161 - Sensitizing acute myeloid leukemia cells to induced differentiation by inhibiting the RIP1/RIP3 pathway.
Xin J, You D, Breslin P, Li J, Zhang J, Wei W, Cannova J, Volk A, Gutierrez R, Xiao Y, Ni A, Ng G, Schmidt R, Xia Z, Pan J, Chen H, Patel MM, Kuo PC, Nand S, Kini AR, Zhang J, Chen J, Zhu J, Zhang J. Xin J, et al. Leukemia. 2017 May;31(5):1154-1165. doi: 10.1038/leu.2016.287. Epub 2016 Oct 17. Leukemia. 2017. PMID: 27748372 Free PMC article. - Smac mimetic LCL161 supports neuroblastoma chemotherapy in a drug class-dependent manner and synergistically interacts with ALK inhibitor TAE684 in cells with ALK mutation F1174L.
Najem S, Langemann D, Appl B, Trochimiuk M, Hundsdoerfer P, Reinshagen K, Eschenburg G. Najem S, et al. Oncotarget. 2016 Nov 8;7(45):72634-72653. doi: 10.18632/oncotarget.12055. Oncotarget. 2016. PMID: 27655666 Free PMC article. - Combining the SMAC mimetic LCL161 with Gemcitabine plus Cisplatin therapy inhibits and prevents the emergence of multidrug resistance in cholangiocarcinoma.
Prasopporn S, Suppramote O, Ponvilawan B, Jamyuang C, Chanthercrob J, Chaiboonchoe A, More-Krong P, Kongsri K, Suntiparpluacha M, Chanwat R, Korphaisarn K, Okada S, Sampattavanich S, Jirawatnotai S. Prasopporn S, et al. Front Oncol. 2022 Nov 30;12:1021632. doi: 10.3389/fonc.2022.1021632. eCollection 2022. Front Oncol. 2022. PMID: 36531039 Free PMC article.
References
- Druker BJ, Tamura S, Buchdunger E, Ohno S, Segal GM, Fanning S, et al. Effects of a selective inhibitor of the Abl tyrosine kinase on the growth of Bcr-Abl positive cells. Nat Med. 1996;2:561–566. - PubMed
- Buchdunger E, Matter A, Druker BJ. Bcr-Abl inhibition as a modality of CML therapeutics. Biochim Biophys Acta. 2001;1551:M11–M18. - PubMed
- Deininger MW, Goldman JM, Melo JV. The molecular biology of chronic myeloid leukemia. Blood. 2000;96:3343–3356. - PubMed
- Gorre ME, Mohammed M, Ellwood K, Hsu N, Paquette R, Rao PN, et al. Clinical resistance to STI-571 cancer therapy caused by BCR-ABL gene mutation or amplification. Science. 2001;293:876–880. - PubMed
- Weisberg E, Griffin JD. Mechanism of resistance to the ABL tyrosine kinase inhibitor STI571 in BCR/ABL-transformed hematopoietic cell lines. Blood. 2000;95:3498–505. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- CA66996/CA/NCI NIH HHS/United States
- CA36167/CA/NCI NIH HHS/United States
- P01 CA066996/CA/NCI NIH HHS/United States
- P01 DK050654/DK/NIDDK NIH HHS/United States
- R01 CA036167/CA/NCI NIH HHS/United States
- R37 CA036167/CA/NCI NIH HHS/United States
- DK50654/DK/NIDDK NIH HHS/United States
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
Miscellaneous