Lenalidomide enhances antibody-dependent cellular cytotoxicity of solid tumor cells in vitro: influence of host immune and tumor markers - PubMed (original) (raw)
Comparative Study
Lenalidomide enhances antibody-dependent cellular cytotoxicity of solid tumor cells in vitro: influence of host immune and tumor markers
Lei Wu et al. Cancer Immunol Immunother. 2011 Jan.
Abstract
We evaluated the effect of combining lenalidomide with therapeutic antibodies on antibody-dependant cell-mediated cytotoxicity (ADCC) of solid tumor cells, and the requirement for expression of natural killer (NK) cell-activating receptors and their solid tumor surface ligands. Twenty-three human tumor cell lines (colon, breast, lung, head and neck, ovary, and bone sarcoma) were analyzed. NK effector cells were isolated from healthy donors, pre-treated with and without lenalidomide, and incubated with antibody-coated tumor cells to determine ADCC. In blocking experiments, NK cells were pre-incubated with anti-DNAM-1 or anti-NKG2D antibodies, and target colorectal cells were pre-incubated with anti-CD155 (PVR), anti-MIC-A/B, or anti-ULBP 3 antibodies. Differences between groups were assessed using unpaired and paired Student's t test and one-way ANOVA. Lenalidomide enhanced NK cell-mediated ADCC of trastuzumab- and cetuximab-coated tumor cells. Activity against colorectal cancer cells was dependent on target antigen expression, but independent of KRAS status and FcγRIIIa genotype. The extent of ADCC and its enhancement by lenalidomide correlated with NK cell expression of NKG2D and DNAM-1, and tumor cell expression of PVR and MIC-A. Blocking of NKG2D and, to a lesser extent, DNAM-1 inhibited ADCC. Anti-MIC-A/B monoclonal antibody blocked natural cytotoxicity, but not ADCC. Lenalidomide enhances the ability of IgG1-isotype antibodies to mediate ADCC of solid tumor cells, the extent of which is largely dependent on NKG2D-NKG2D ligand interactions, but appears to be independent of MIC-A/B. This provides a rationale for exploratory clinical studies and an assessment of potential biomarkers predictive of clinical benefit.
Conflict of interest statement
All of the authors are employees of Celgene Corporation.
Figures
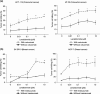
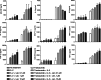
Fig. 1
Dose-dependent enhancement of natural killer (NK) cell-mediated cytotoxicity of antibody-coated tumor cells by lenalidomide: a cetuximab-coated HCT-116 and HT-29 colorectal cancer cells (E:T cell ratio 10:1); and b trastuzumab-coated _SK_-_BR_-3 and _MCF_-7 breast cancer cells (E:T cell ratio 10:1). Data are expressed as % tumor cell killing (mean ± SD of three individual experiments each performed in triplicate). Asterisks indicate significant difference between the killing of antibody-coated target cells versus uncoated target cells, by lenalidomide pre-treated NK cells (*P < 0.05; **P < 0.01; two-way ANOVA with Bonferroni post hoc test)
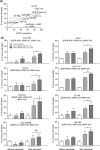
Fig. 2
a EGFR expression levels in colorectal cancer (CRC) cell lines are associated with the ability of lenalidomide (LEN) to enhance natural killer (NK) cell-mediated antibody-dependant cell-mediated cytotoxicity (ADCC). b CRC KRAS and BRAF mutational status does not affect the ability of lenalidomide to enhance NK cell-mediated ADCC. CRC cell lines expressing different levels of EGFR and/or harboring KRAS or BRAF mutations were used as NK cell targets in a 4-h ADCC assay. Purified NK cells were pre-treated with lenalidomide (1 μM) overnight and added to cetuximab pre-treated CRC cell targets at an E:T cell ratio of 10:1. Data shown are the average of six individual experiments and expressed as % tumor cell killing (mean ± SD). Asterisks indicate significant difference between the killing of cetuximab-coated CRC cells by untreated versus lenalidomide pre-treated NK cells (*P < 0.05; **P < 0.01; one-way ANOVA test) Ab antibody, EGFR epidermal growth factor receptor, mut mutant, NS not significant, WT wild-type
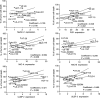
Fig. 3
Effect of colorectal cancer (CRC) cell surface expression of DNAX accessory molecule-1 and natural killer (NK) group 2 member D ligands on sensitivity to NK cell-mediated antibody-dependant cell-mediated cytotoxicity. The results show the influence of tumor cell expression of Nectin-2, poliovirus receptor (PVR), MHC class I-related chain (MIC)-A/B, UL-16-binding proteins (ULBP) 2, and ULBP 3 on the ability of lenalidomide pre-treated NK cells to kill the CRC cells tested. The data are expressed as a % of tumor cell death versus ligand expression (defined as ratio of the mean fluorescence intensity) in each CRC cell line and are from six separate experiments
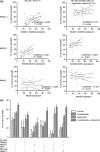
Fig. 4
The influence of FcγRIIIa genotype on the ability of lenalidomide to enhance antibody-dependant cell-mediated cytotoxicity of colorectal cancer cells. Natural killer (NK) cells from 30 and 24 healthy donors were used as effector cells against a cetuximab-coated HCT-116 cells and b trastuzumab-coated SK-BR-3 cells respectively (E:T cell ratio 10:1). NK cells were grouped into donors with at least one copy of the high-affinity FcγRIIIa V allele (158 VV or 158 VF) and donors with two copies of the FcγRIIIa low-affinity F allele (158 FF). Data show % baseline tumor cell killing (mean ± SD), the effect of adding NK cells, and the additional effect of lenalidomide pre-treated NK cells on untreated and cetuximab- or trastuzumab-coated tumor cells
Fig. 5
Influence of surface expression of DNAX accessory molecule (DNAM)-1 and natural killer (NK) group 2 member D (NKG2D), and their interaction with ligands on the tumor-cell surface on ADCC enhancement by lenalidomide. a Analysis of the activating receptors involved in the lysis of solid tumors. FACS analysis of antibody-dependant cell-mediated cytotoxicity (ADCC), DNAM-1, NKG2D, and NKp46 expression in NK cells from 30 healthy donors. The correlation analysis of DNAM-1, NKG2D, and NKp46 expression levels (defined as ratio of the mean fluorescence intensity) in NK cell-mediated antibody-dependant cell-mediated cytotoxicity against cetuximab-coated HCT-116 in a 4-h lactate dehydrogenase release cytotoxicity assay (E:T cell ratio 10:1). b Efficient natural killer (NK) cell-mediated killing of colorectal cancer (CRC) cells and the enhancement of ADCC by lenalidomide requires efficient interaction between NKG2D on the NK cell surface and its ligands on the tumor surface. Untreated or lenalidomide pre-treated NK cells were assessed for the ability to kill uncoated and cetuximab-coated CRC cells (HCT-116) (E:T cell ratio 20:1). In parallel cultures, the effect of pre-treatment of NK cells with saturating amounts of blocking antibodies to either DNAM-1 or NKG2D, or the effect of pre-treatment of tumor cells with saturating amounts of blocking antibodies to CD155, MHC class I-related chain (MIC)-A/B, or UL-16 binding proteins (ULBP) 3 was assessed. Data are expressed as % of lysis (mean ± SD) using NK cells from three healthy donors. Asterisks indicate significant inhibition of killing by specific blocking antibody compared with identical culture in the absence of antibody (*P < 0.05; **P < 0.01; paired Student’s t test) mAb monoclonal antibody, PVR poliovirus receptor
Fig. 6
Lenalidomide (Len) enhances the production of natural killer (NK) cell-derived inflammatory cytokines and chemokines in response to trastuzumab-coated SK-BR-3 breast cancer cells in vitro. Trastuzumab-coated SK-BR-3 tumor cells co-incubated with NK cells for 48 h in response to lenalidomide, cell-free culture supernatants were harvested and analyzed for cytokine and chemokine levels using Luminex and commercially validated kits as described in “Materials and methods”. Asterisks indicate significant enhancement by lenalidomide (mean ± SD; *P < 0.05; **P < 0.01; using data from three separate experiments) compared with supernatants derived from control cultures
Similar articles
- Taxanes enhance trastuzumab-mediated ADCC on tumor cells through NKG2D-mediated NK cell recognition.
Di Modica M, Sfondrini L, Regondi V, Varchetta S, Oliviero B, Mariani G, Bianchi GV, Generali D, Balsari A, Triulzi T, Tagliabue E. Di Modica M, et al. Oncotarget. 2016 Jan 5;7(1):255-65. doi: 10.18632/oncotarget.6353. Oncotarget. 2016. PMID: 26595802 Free PMC article. - lenalidomide enhances natural killer cell and monocyte-mediated antibody-dependent cellular cytotoxicity of rituximab-treated CD20+ tumor cells.
Wu L, Adams M, Carter T, Chen R, Muller G, Stirling D, Schafer P, Bartlett JB. Wu L, et al. Clin Cancer Res. 2008 Jul 15;14(14):4650-7. doi: 10.1158/1078-0432.CCR-07-4405. Clin Cancer Res. 2008. PMID: 18628480 - Ex vivo antibody-dependent cellular cytotoxicity inducibility predicts efficacy of cetuximab.
Taylor RJ, Saloura V, Jain A, Goloubeva O, Wong S, Kronsberg S, Nagilla M, Silpino L, de Souza J, Seiwert T, Vokes E, Villaflor V, Cohen EE. Taylor RJ, et al. Cancer Immunol Res. 2015 May;3(5):567-74. doi: 10.1158/2326-6066.CIR-14-0188. Epub 2015 Mar 13. Cancer Immunol Res. 2015. PMID: 25769300 Free PMC article. Clinical Trial. - Mechanisms of NK Cell Activation and Clinical Activity of the Therapeutic SLAMF7 Antibody, Elotuzumab in Multiple Myeloma.
Campbell KS, Cohen AD, Pazina T. Campbell KS, et al. Front Immunol. 2018 Nov 5;9:2551. doi: 10.3389/fimmu.2018.02551. eCollection 2018. Front Immunol. 2018. PMID: 30455698 Free PMC article. Review. - Effect of NKG2D ligand expression on host immune responses.
Champsaur M, Lanier LL. Champsaur M, et al. Immunol Rev. 2010 May;235(1):267-85. doi: 10.1111/j.0105-2896.2010.00893.x. Immunol Rev. 2010. PMID: 20536569 Free PMC article. Review.
Cited by
- Combination strategies to enhance antitumor ADCC.
Kohrt HE, Houot R, Marabelle A, Cho HJ, Osman K, Goldstein M, Levy R, Brody J. Kohrt HE, et al. Immunotherapy. 2012 May;4(5):511-27. doi: 10.2217/imt.12.38. Immunotherapy. 2012. PMID: 22642334 Free PMC article. Review. - Incidence and risk of hypomagnesemia in advanced cancer patients treated with cetuximab: A meta-analysis.
Chen P, Wang L, Li H, Liu B, Zou Z. Chen P, et al. Oncol Lett. 2013 Jun;5(6):1915-1920. doi: 10.3892/ol.2013.1301. Epub 2013 Apr 4. Oncol Lett. 2013. PMID: 23833666 Free PMC article. - Clinical and Immune Effects of Lenalidomide in Combination with Gemcitabine in Patients with Advanced Pancreatic Cancer.
Ullenhag GJ, Mozaffari F, Broberg M, Mellstedt H, Liljefors M. Ullenhag GJ, et al. PLoS One. 2017 Jan 18;12(1):e0169736. doi: 10.1371/journal.pone.0169736. eCollection 2017. PLoS One. 2017. PMID: 28099502 Free PMC article. Clinical Trial. - Prospects for NK Cell Therapy of Sarcoma.
Lachota M, Vincenti M, Winiarska M, Boye K, Zagożdżon R, Malmberg KJ. Lachota M, et al. Cancers (Basel). 2020 Dec 11;12(12):3719. doi: 10.3390/cancers12123719. Cancers (Basel). 2020. PMID: 33322371 Free PMC article. Review. - From Friend to Enemy: Dissecting the Functional Alteration of Immunoregulatory Components during Pancreatic Tumorigenesis.
Wang HC, Hung WC, Chen LT, Pan MR. Wang HC, et al. Int J Mol Sci. 2018 Nov 13;19(11):3584. doi: 10.3390/ijms19113584. Int J Mol Sci. 2018. PMID: 30428588 Free PMC article. Review.
References
- Fowler NH, McLaughlin P, Kwak L, Hagemeister F, Fanale M, Fayad L, Pro B, Samaniego F. Lenalidomide and rituximab for untreated indolent non-Hodgkin’s lymphoma [abstract] J Clin Oncol. 2009;27(15s):8548.
- Veliz M, Santana R, Lancet JE, Komrokji RS, Kharfan-Dabaja MA, Powers JJ, Dubovsky JA, Tinsley S, Deaver D, Sotomayor EM, Pinilla-Ibarz J. Phase II study of lenalidomide in combination with rituximab for patients with CD5+/CD20+ hematologic malignancies who relapse or progress after rituximab. interim analysis [abstract] Blood. 2009;114:2376.
- Ahmadi T, Chong EA, Gordon A, Aqui NA, Downs LH, Leinbach L, Goldstein SC, Nasta SD, Janofsky S, Maniar TN, Svoboda J, Schuster SJ. Phase II trial of lenalidomide–dexamethasone–rituximab in relapsed or refractory indolent B-cell or mantle cell lymphomas resistant to rituximab [abstract] Blood. 2009;114:1700.
- Kalmadi S, Davis M, Dowlati A, O’Keefe S, Cline-Burkhardt M, Pelley RJ, Borden E, Dreicer R, Bukowski R, Mekhail T. Phase I trial of three-weekly docetaxel, carboplatin and oral lenalidomide (Revlimid) in patients with advanced solid tumors. Invest New Drugs. 2007;25:211–216. doi: 10.1007/s10637-006-9025-4. - DOI - PubMed
- Petrylak DP, Resto-Garces K, Tibyan M, Mohile SG. A phase I open-label study using lenalidomide and docetaxel in castration- resistant prostate cancer [abstract] J Clin Oncol. 2009;27(15s):5156.
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
Miscellaneous