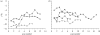Stable isotopes in fossil hominin tooth enamel suggest a fundamental dietary shift in the Pliocene - PubMed (original) (raw)
Review
Stable isotopes in fossil hominin tooth enamel suggest a fundamental dietary shift in the Pliocene
Julia A Lee-Thorp et al. Philos Trans R Soc Lond B Biol Sci. 2010.
Abstract
Accumulating isotopic evidence from fossil hominin tooth enamel has provided unexpected insights into early hominin dietary ecology. Among the South African australopiths, these data demonstrate significant contributions to the diet of carbon originally fixed by C(4) photosynthesis, consisting of C(4) tropical/savannah grasses and certain sedges, and/or animals eating C(4) foods. Moreover, high-resolution analysis of tooth enamel reveals strong intra-tooth variability in many cases, suggesting seasonal-scale dietary shifts. This pattern is quite unlike that seen in any great apes, even 'savannah' chimpanzees. The overall proportions of C(4) input persisted for well over a million years, even while environments shifted from relatively closed (ca 3 Ma) to open conditions after ca 1.8 Ma. Data from East Africa suggest a more extreme scenario, where results for Paranthropus boisei indicate a diet dominated (approx. 80%) by C(4) plants, in spite of indications from their powerful 'nutcracker' morphology for diets of hard objects. We argue that such evidence for engagement with C(4) food resources may mark a fundamental transition in the evolution of hominin lineages, and that the pattern had antecedents prior to the emergence of Australopithecus africanus. Since new isotopic evidence from Aramis suggests that it was not present in Ardipithecus ramidus at 4.4 Ma, we suggest that the origins lie in the period between 3 and 4 Myr ago.
Figures
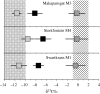
Figure 1.
Data for all the South African hominins are summarized as means _δ_13C (black boxes) and standard deviations compared with means and standard deviations for the browsing and grazing fauna. The sites are given in sequence from oldest (top) to youngest (bottom). Makapansgat Member 3 is about 2.7–3 Ma, Sterkfontein Member 4 is usually considered to be about 2.2–2.5 Ma and Swartkrans Member 1 is younger than 2 Ma. These ages based on biostratigraphy are imprecise, but sufficient for our purposes. More precise chronometric studies based on Pb/U isotopes have recently been completed (R. Pickering 2009, personal communication), but do not change the overall sequence. All the hominin data show significant C4 contributions compared with C3 feeders, in spite of large shifts, from closed to open, in the environments (Reed 1997). Adapted from Lee-Thorp & Sponheimer (2006).
Figure 2.
High-resolution laser ablation δ_13C sequences for (a) A. africanus and (b) P. robustus plotted against sample (scan) number. Sample increments were approximately 0.3 mm. The Paranthropus data are from Sponheimer et al. (2006_b), where the data were plotted according to a time-sequence model based on perikymata counts. In this case, we avoided the application of a time sequence based on perikymata because the lengthy maturation time introduces not only a longer time period but also more uncertainty. (a) Open diamonds, STS 2518 max RM3; filled circles, STS 31 max RM3; filled triangles, STS 2253 mand RM1. (b) Open diamonds, SKW 6427 M; filled circles, SKW 5939 M; filled triangles, SK 24606 RM2 or 3; squares with crosses, SK 24606 RM3.
Figure 3.
A summary comparison of the hominin data for Aramis (above), and Makapansgat (below), plotted as means (black boxes) and standard deviations with mean values for C3 and C4 feeders shown for comparison. Data for carnivores (hyaenids) are also shown (white boxes; n = 2 for Makapansgat), because they are effectively integrators for the values of all the fauna they consume. These data are shifted towards values more enriched in 13C in Aramis, suggesting more open, C4 elements in the environment compared with Makapansgat, where the faunal assemblage consists largely of C3 feeders. In spite of this difference, Ar. ramidus remains relatively depleted in 13C, quite unlike the patterns for A. africanus seen at Makapansgat. Data for Aramis are from White et al. (2009_b_), and for Makapansgat are from Sponheimer (1999).
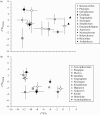
Figure 4.
Bivariate _δ_13C and _δ_18O comparisons of similar taxa in (a) Aramis and (b) Makapansgat shown as means and standard deviations. Several significant differences are observed. On average, _δ_18O values for Makapansgat fauna are about 2‰ lower than at Aramis, which is consistent with their relative geographical positions and associated values for hydrology. However, the Aramis data are more variable, with some exceptional and unusually low values for Deinotherium in particular. Australopithecus africanus data are relatively enriched in 13C and depleted in 18O, and occupy the same isotopic ‘space’ as the hyaenids, quite unlike Ar. ramidus. The other primate species are also more enriched in 13C, unlike Aramis, but the impala (Aepyceros) has higher _δ_13C values at Aramis. Although impalas are generally considered to be mixed feeders, a recent study showed high δ_13C and almost exclusive grazing habits for Aepyceros in the nutritious grasslands of Rwanda (Copeland et al. 2009). The data for Nyanzochoerus at Aramis are remarkably similar to those for the Suidae at Makapansgat, suggesting a similar ecological niche. Data for Aramis are from White et al. (2009_b) and for Makapansgat from Sponheimer (1999).
Similar articles
- Isotopic evidence for an early shift to C₄ resources by Pliocene hominins in Chad.
Lee-Thorp J, Likius A, Mackaye HT, Vignaud P, Sponheimer M, Brunet M. Lee-Thorp J, et al. Proc Natl Acad Sci U S A. 2012 Dec 11;109(50):20369-72. doi: 10.1073/pnas.1204209109. Epub 2012 Nov 12. Proc Natl Acad Sci U S A. 2012. PMID: 23150583 Free PMC article. - The carbon isotope ecology and diet of Australopithecus africanus at Sterkfontein, South Africa.
van der Merwe NJ, Thackeray JF, Lee-Thorp JA, Luyt J. van der Merwe NJ, et al. J Hum Evol. 2003 May;44(5):581-97. doi: 10.1016/s0047-2484(03)00050-2. J Hum Evol. 2003. PMID: 12765619 - Isotopic evidence for dietary variability in the early hominin Paranthropus robustus.
Sponheimer M, Passey BH, de Ruiter DJ, Guatelli-Steinberg D, Cerling TE, Lee-Thorp JA. Sponheimer M, et al. Science. 2006 Nov 10;314(5801):980-2. doi: 10.1126/science.1133827. Science. 2006. PMID: 17095699 - The diets of early hominins.
Ungar PS, Sponheimer M. Ungar PS, et al. Science. 2011 Oct 14;334(6053):190-3. doi: 10.1126/science.1207701. Science. 2011. PMID: 21998380 Review. - Dental evidence for the diets of Plio-Pleistocene hominins.
Ungar PS. Ungar PS. Am J Phys Anthropol. 2011;146 Suppl 53:47-62. doi: 10.1002/ajpa.21610. Am J Phys Anthropol. 2011. PMID: 22101687 Review.
Cited by
- Isotopic evidence for an early shift to C₄ resources by Pliocene hominins in Chad.
Lee-Thorp J, Likius A, Mackaye HT, Vignaud P, Sponheimer M, Brunet M. Lee-Thorp J, et al. Proc Natl Acad Sci U S A. 2012 Dec 11;109(50):20369-72. doi: 10.1073/pnas.1204209109. Epub 2012 Nov 12. Proc Natl Acad Sci U S A. 2012. PMID: 23150583 Free PMC article. - A Major Change in Rate of Climate Niche Envelope Evolution during Hominid History.
Mondanaro A, Melchionna M, Di Febbraro M, Castiglione S, Holden PB, Edwards NR, Carotenuto F, Maiorano L, Modafferi M, Serio C, Diniz-Filho JAF, Rangel T, Rook L, O'Higgins P, Spikins P, Profico A, Raia P. Mondanaro A, et al. iScience. 2020 Oct 15;23(11):101693. doi: 10.1016/j.isci.2020.101693. eCollection 2020 Nov 20. iScience. 2020. PMID: 33163945 Free PMC article. - Unexpectedly rapid evolution of mandibular shape in hominins.
Raia P, Boggioni M, Carotenuto F, Castiglione S, Di Febbraro M, Di Vincenzo F, Melchionna M, Mondanaro A, Papini A, Profico A, Serio C, Veneziano A, Vero VA, Rook L, Meloro C, Manzi G. Raia P, et al. Sci Rep. 2018 May 9;8(1):7340. doi: 10.1038/s41598-018-25309-8. Sci Rep. 2018. PMID: 29743608 Free PMC article. - The discovery of fire by humans: a long and convoluted process.
Gowlett JA. Gowlett JA. Philos Trans R Soc Lond B Biol Sci. 2016 Jun 5;371(1696):20150164. doi: 10.1098/rstb.2015.0164. Philos Trans R Soc Lond B Biol Sci. 2016. PMID: 27216521 Free PMC article. Review. - Changing perspectives on early hominin diets.
Teaford MF, Ungar PS, Grine FE. Teaford MF, et al. Proc Natl Acad Sci U S A. 2023 Feb 14;120(7):e2201421120. doi: 10.1073/pnas.2201421120. Epub 2023 Feb 6. Proc Natl Acad Sci U S A. 2023. PMID: 36745809 Free PMC article.
References
- Balasse M.2002Reconstructing dietary and environmental history from enamel isotopic analysis: time resolution of intra-tooth sequential sampling. Int. J. Osteoarch. 12, 155–165 (doi:10.1002/oa.601) - DOI
- Bocherens H., Koch P. L., Mariotti A., Geraads D., Jaeger J.1996Isotopic biogeochemistry (13C, 18O) of mammalian enamel from African Pleistocene hominid sites. PALAIOS 11, 306–318 (doi:10.2307/3515241) - DOI
- Carter M. L.2001Sensitivity of stable isotopes (13C, 15N, and 18O) in bone to dietary specialization and niche separation among sympatric primates in Kibale National Park, Uganda. PhD dissertation, University of Chicago
- Cerling T. E., Harris J. M., MacFadden B. J., Leakey M. G., Quade J., Eisenman V., Ehleringer J. R.1997Global vegetation change through the Miocene/Pliocene boundary. Nature 389, 153–158 (doi:10.1038/38229) - DOI
- Cerling T. E., Hart J. A., Hart T. B.2004Stable isotope ecology in the Ituri Forest. Oecologia 138, 5–12 (doi:10.1007/s00442-003-1375-4) - DOI - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Miscellaneous