Fragile X and autism: Intertwined at the molecular level leading to targeted treatments - PubMed (original) (raw)
Fragile X and autism: Intertwined at the molecular level leading to targeted treatments
Randi Hagerman et al. Mol Autism. 2010.
Abstract
Fragile X syndrome (FXS) is caused by an expanded CGG repeat (> 200 repeats) in the 5' untranslated portion of the fragile mental retardation 1 gene (FMR1), leading to deficiency or absence of the FMR1 protein (FMRP). FMRP is an RNA carrier protein that controls the translation of several other genes that regulate synaptic development and plasticity. Autism occurs in approximately 30% of FXS cases, and pervasive developmental disorder, not otherwise specified (PDD-NOS) occurs in an additional 30% of cases. Premutation repeat expansions (55 to 200 CGG repeats) may also give rise to autism spectrum disorders (ASD), including both autism and PDD-NOS, through a different molecular mechanism that involves a direct toxic effect of the expanded CGG repeat FMR1 mRNA. RNA toxicity can also lead to aging effects including tremor, ataxia and cognitive decline, termed fragile X-associated tremor ataxia syndrome (FXTAS), in premutation carriers in late life. In studies of mice bearing premutation expansions, there is evidence of early postnatal neuronal cell toxicity, presenting as reduced cell longevity, decreased dendritic arborization and altered synaptic morphology. There is also evidence of mitochondrial dysfunction in premutation carriers. Many of the problems with cellular dysregulation in both premutation and full mutation neurons also parallel the cellular abnormalities that have been documented in autism without fragile X mutations. Research regarding dysregulation of neurotransmitter systems in FXS, including the metabotropic glutamate receptor (mGluR)1/5 pathway and γ aminobutyric acid (GABA)A pathways, have led to new targeted treatments for FXS. Preliminary evidence suggests that these new targeted treatments will also be beneficial in non-fragile X forms of autism.
Figures
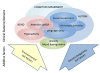
Figure 1
Overview of the behavioral/cognitive phenotype of fragile X syndrome (FXS). The interrelationships among cognitive, behavioral and attentional deficits in FXS are modified by additional environmental influences and genetic background effects. Environmental influences include seizures, trauma, abuse and socioeconomic status. Genetic influences include allelic variations, additional genetic disorders and variation in the expression levels of genes important for the phenotype of FXS.
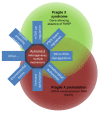
Figure 2
Molecular overlap between autism, fragile x syndrome (FXS) and premutation disorders. Absence of the FMR1 protein (FMRP) leads to the dysregulation of several proteins including those involved with synapse formation and plasticity, glutamate and γ aminobuyric acid (GABA) neurotransmission and mammalian target of rapamycin (mTOR) and phosphatase and tensin homolog (PTEN) pathways. A premutation is associated with elevation of FMR1 mRNA, leading to sequestration of proteins and mitochondrial dysfunction. Many of these same molecular changes can also occur in some types of autism. Some patients with FXS have mosaicism of premutation and full cells, so there is overlap of the molecular mechanisms among all three disorders.
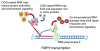
Figure 3
Potential mechanisms of FMR1 mRNA toxicity. Although numerous studies point to RNA toxicity as the underlying pathogenic trigger in fragile X-associated tremor ataxia syndrome (FXTAS), the specific mechanism for such toxicity is not known. Possibilities include (1) sequestration of one or more proteins that bind to the RNA, thus attenuating their other cell functions; (2) protein activation upon binding to the CGG-repeat RNA, leading to dysregulation of one or more signaling cascades; and (3) various co-transcriptional process, such as R-loop formation, that lead to DNA damage/repair signaling and consequent cellular dysregulation.
Similar articles
- FMR1 premutation and full mutation molecular mechanisms related to autism.
Hagerman R, Au J, Hagerman P. Hagerman R, et al. J Neurodev Disord. 2011 Sep;3(3):211-24. doi: 10.1007/s11689-011-9084-5. Epub 2011 May 27. J Neurodev Disord. 2011. PMID: 21617890 Free PMC article. - Impaired activity-dependent FMRP translation and enhanced mGluR-dependent LTD in Fragile X premutation mice.
Iliff AJ, Renoux AJ, Krans A, Usdin K, Sutton MA, Todd PK. Iliff AJ, et al. Hum Mol Genet. 2013 Mar 15;22(6):1180-92. doi: 10.1093/hmg/dds525. Epub 2012 Dec 18. Hum Mol Genet. 2013. PMID: 23250915 Free PMC article. - Fragile X: leading the way for targeted treatments in autism.
Wang LW, Berry-Kravis E, Hagerman RJ. Wang LW, et al. Neurotherapeutics. 2010 Jul;7(3):264-74. doi: 10.1016/j.nurt.2010.05.005. Neurotherapeutics. 2010. PMID: 20643379 Free PMC article. Review. - Unstable mutations in the FMR1 gene and the phenotypes.
Loesch D, Hagerman R. Loesch D, et al. Adv Exp Med Biol. 2012;769:78-114. doi: 10.1007/978-1-4614-5434-2_6. Adv Exp Med Biol. 2012. PMID: 23560306 Free PMC article. Review. - Fragile X syndrome and fragile X-associated tremor ataxia syndrome.
Hall DA, Berry-Kravis E. Hall DA, et al. Handb Clin Neurol. 2018;147:377-391. doi: 10.1016/B978-0-444-63233-3.00025-7. Handb Clin Neurol. 2018. PMID: 29325626 Review.
Cited by
- Evidence of a distinct behavioral phenotype in young boys with fragile X syndrome and autism.
Wolff JJ, Bodfish JW, Hazlett HC, Lightbody AA, Reiss AL, Piven J. Wolff JJ, et al. J Am Acad Child Adolesc Psychiatry. 2012 Dec;51(12):1324-32. doi: 10.1016/j.jaac.2012.09.001. J Am Acad Child Adolesc Psychiatry. 2012. PMID: 23200289 Free PMC article. - Progress toward therapeutic potential for AFQ056 in Fragile X syndrome.
Sourial M, Cheng C, Doering LC. Sourial M, et al. J Exp Pharmacol. 2013 Jul 16;5:45-54. doi: 10.2147/JEP.S27044. eCollection 2013. J Exp Pharmacol. 2013. PMID: 27186135 Free PMC article. Review. - Identification of expanded alleles of the FMR1 gene among high-risk population in Indonesia by using blood spot screening.
Winarni TI, Utari A, Mundhofir FE, Tong T, Durbin-Johnson B, Faradz SM, Tassone F. Winarni TI, et al. Genet Test Mol Biomarkers. 2012 Mar;16(3):162-6. doi: 10.1089/gtmb.2011.0089. Epub 2011 Oct 11. Genet Test Mol Biomarkers. 2012. PMID: 21988366 Free PMC article. - BC RNA Mislocalization in the Fragile X Premutation.
Muslimov IA, Eom T, Iacoangeli A, Chuang SC, Hukema RK, Willemsen R, Stefanov DG, Wong RKS, Tiedge H. Muslimov IA, et al. eNeuro. 2018 Apr 19;5(2):ENEURO.0091-18.2018. doi: 10.1523/ENEURO.0091-18.2018. eCollection 2018 Mar-Apr. eNeuro. 2018. PMID: 29766042 Free PMC article. - Of Men and Mice: Modeling the Fragile X Syndrome.
Dahlhaus R. Dahlhaus R. Front Mol Neurosci. 2018 Mar 15;11:41. doi: 10.3389/fnmol.2018.00041. eCollection 2018. Front Mol Neurosci. 2018. PMID: 29599705 Free PMC article.
References
- Miller DT, Adam MP, Aradhya S, Biesecker LG, Brothman AR, Carter NP, Church DM, Crolla JA, Eichler EE, Epstein CJ, Faucett WA, Feuk L, Friedman JM, Hamosh A, Jackson L, Kaminsky EB, Kok K, Krantz ID, Kuhn RM, Lee C, Ostell JM, Rosenberg C, Scherer SW, Spinner NB, Stavropoulos DJ, Tepperberg JH, Thorland EC, Vermeesch JR, Waggoner DJ, Watson MS, Martin CL, Ledbetter DH. Consensus statement: chromosomal microarray is a first-tier clinical diagnostic test for individuals with developmental disabilities or congenital anomalies. Am J Hum Genet. 2010;86:749–764. doi: 10.1016/j.ajhg.2010.04.006. - DOI - PMC - PubMed
- Nishimura Y, Martin CL, Vazquez-Lopez A, Spence SJ, Alvarez-Retuerto AI, Sigman M, Steindler C, Pellegrini S, Schanen NC, Warren ST, Geschwind DH. Genome-wide expression profiling of lymphoblastoid cell lines distinguishes different forms of autism and reveals shared pathways. Hum Mol Genet. 2007;16:1682–1698. doi: 10.1093/hmg/ddm116. - DOI - PubMed
LinkOut - more resources
Full Text Sources
Other Literature Sources