NLRP3 inflammasome plays a key role in the regulation of intestinal homeostasis - PubMed (original) (raw)
. 2011 Jun;17(6):1359-72.
doi: 10.1002/ibd.21478. Epub 2010 Sep 24.
Jeffrey Ng, Alan Lueng, Maitham Khajah, Ken Parhar, Yan Li, Victor Lam, Mireille S Potentier, Kelvin Ng, Misha Bawa, Donna-Marie McCafferty, Kevin P Rioux, Subrata Ghosh, Ramnik J Xavier, Sean P Colgan, Jurg Tschopp, Daniel Muruve, Justin A MacDonald, Paul L Beck
Affiliations
- PMID: 20872834
- PMCID: PMC3026862
- DOI: 10.1002/ibd.21478
NLRP3 inflammasome plays a key role in the regulation of intestinal homeostasis
Simon A Hirota et al. Inflamm Bowel Dis. 2011 Jun.
Abstract
Background: Attenuated innate immune responses to the intestinal microbiota have been linked to the pathogenesis of Crohn's disease (CD). Recent genetic studies have revealed that hypofunctional mutations of NLRP3, a member of the NOD-like receptor (NLR) superfamily, are associated with an increased risk of developing CD. NLRP3 is a key component of the inflammasome, an intracellular danger sensor of the innate immune system. When activated, the inflammasome triggers caspase-1-dependent processing of inflammatory mediators, such as IL-1β and IL-18.
Methods: In the current study we sought to assess the role of the NLRP3 inflammasome in the maintenance of intestinal homeostasis through its regulation of innate protective processes. To investigate this role, Nlrp3(-/-) and wildtype mice were assessed in the dextran sulfate sodium and 2,4,6-trinitrobenzenesulfonic acid models of experimental colitis.
Results: Nlrp3(-/-) mice were found to be more susceptible to experimental colitis, an observation that was associated with reduced IL-1β, reduced antiinflammatory cytokine IL-10, and reduced protective growth factor TGF-β. Macrophages isolated from Nlrp3(-/-) mice failed to respond to bacterial muramyl dipeptide. Furthermore, Nlrp3-deficient neutrophils exhibited reduced chemotaxis and enhanced spontaneous apoptosis, but no change in oxidative burst. Lastly, Nlrp3(-/-) mice displayed altered colonic β-defensin expression, reduced colonic antimicrobial secretions, and a unique intestinal microbiota.
Conclusions: Our data confirm an essential role for the NLRP3 inflammasome in the regulation of intestinal homeostasis and provide biological insight into disease mechanisms associated with increased risk of CD in individuals with NLRP3 mutations.
Copyright © 2010 Crohn's & Colitis Foundation of America, Inc.
Figures
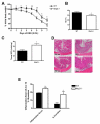
Figure 1
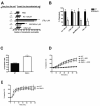
Nlrp3−/− mice exhibit enhanced susceptibility to DSS-induced colitis as exhibited by accelerated weight loss (A); reduced hematocrit (B); increased colonic MPO (C). Nlrp3−/− mice exposed to DSS exhibited significantly more inflammation, elevated damage score and overall magnitude of ulceration (D-E; Inflammation score ×10), which is evident in colonic sections stained with H&E. N=8-12; * denotes p<0.05 compared to WT mice; ** denotes p<0.005 compared to WT mice.
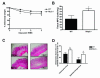
Figure 2
Nlrp3−/− mice exhibit enhanced susceptibility to TNBS-induced colitis as exhibited by accelerated weight loss (A) and increased colonic MPO (B). Nlrp3−/− mice exposed to TNBS exhibited significantly more inflammation and elevated damage which is evident in colonic sections stained with H&E (C) and quantified by blinded histological assessment (D). N=8/group; * denotes p<0.05 compared to WT mice.
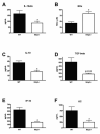
Figure 3
Cytokine, chemokine and NOx levels are altered in Nlrp3−/− mice exposed to a 7-day course of DSS colitis when compared to WT mice. N=3-8/group; * denotes p<0.05 compared to levels observed in WT mice.
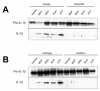
Figure 4
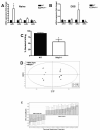
Peritoneal macrophages isolated from Nlrp3-/- mice fail to respond to bacterial muramyl dipeptide (A) Macrophages pre-treated with ultra-pure lipopolysaccharide (LPS, 10ng/mL for 1h) exhibit IL-1β processing and release into culture media when exposed (6h) to muramyl dipeptide (MDP, 10μg/mL), monosodium urate crystals (MSU, 50μg/mL), aluminum adjuvant (Alum, 500μg/mL) and adenosine triphospate (ATP, 5mM). IL-1β processing is inhibited by glyburide (100μM), a selective inhibitor of the NLRP3-inflammasome, and absent in NLRP3-deficient macrophages (B). Western blot are representative of 4 separate experiments.
Figure 5
Chemotaxis and spontaneous apoptosis are altered in BM-derived neutrophils isolated from Nlrp3−/− mice. (A) Overall neutrophil movement in response to PBS (vehicle control), KC (1.25 μM), leukotriene B4 (1 μM), WKTMVm (1 μM) and C5a (1 μM) as assessed with an under-agarose assay. (B) Neutrophil chemotaxis expressed as the number of cells that migrated towards the chemoattractant as a percentage of the total number of cells that moved in any direction. N=4; * denotes p<0.05 compared to WT cells. (C) Spontaneous apoptosis of BM-derived neutrophils after 12h in culture as assessed by flow cytometry. N=4; * denotes p<0.05 compared to WT cells. (D-E) Assessment of PMA(8 μM; D)- and fMLP(8 mM; E)-induced superoxide production in BM-derived neutrophils isolated from WT and Nlrp3−/− mice. N=4, * denotes p<0.05 compared to WT cells. Addition of superoxide dismutase (SOD, 1500 U) was employed as a control to quench superoxide accumulation (D).
Figure 6
Nlrp3−/− mice display altered expression of β-defensin transcripts and exhibit a unique intestinal microbiota. (A) qPCR assessment of β-defensin transcripts in naïve WT and Nlrp3−/− mice expressed as a fold-change. (B) qPCR assessment of β-defensin transcripts in DSS-treated WT and Nlrp3−/− mice expressed as a fold-change (7-day course of 2.5% DSS). _defb1_–mouse β-defensin 1; _defb2_–mouse β-defensin 2; _defb3_–mouse β-defensin 3; _defb4_–mouse β-defensin 4. * denotes p<0.05 compared to naïve WT mice; N=5/group. (C) The antimicrobial capacity of crypt secretions derived from WT and Nlrp3−/− mice. Overnight colony growth in secretion-treated E. coli cultures was compared to untreated cultures and expressed as percent reduction in colony numbers, termed percent killing. * denotes p<0.05 compared to WT mice; N=3. (D) Supervised OPL-S discriminant analysis of TRFLP binary data shows that naïve Nlrp3−/− mice display a unique microbiota when compared to WT littermates. (E) Several unique TRFs were found to significantly distinguish the two groups. N=7/group for TRFLP analysis.
Similar articles
- Colitis induced in mice with dextran sulfate sodium (DSS) is mediated by the NLRP3 inflammasome.
Bauer C, Duewell P, Mayer C, Lehr HA, Fitzgerald KA, Dauer M, Tschopp J, Endres S, Latz E, Schnurr M. Bauer C, et al. Gut. 2010 Sep;59(9):1192-9. doi: 10.1136/gut.2009.197822. Epub 2010 May 4. Gut. 2010. PMID: 20442201 - Protective and aggravating effects of Nlrp3 inflammasome activation in IBD models: influence of genetic and environmental factors.
Bauer C, Duewell P, Lehr HA, Endres S, Schnurr M. Bauer C, et al. Dig Dis. 2012;30 Suppl 1:82-90. doi: 10.1159/000341681. Epub 2012 Oct 11. Dig Dis. 2012. PMID: 23075874 - Genetic and Epigenetic Regulation of the Innate Immune Response to Gout.
de Lima JD, de Paula AGP, Yuasa BS, de Souza Smanioto CC, da Cruz Silva MC, Dos Santos PI, Prado KB, Winter Boldt AB, Braga TT. de Lima JD, et al. Immunol Invest. 2023 Apr;52(3):364-397. doi: 10.1080/08820139.2023.2168554. Epub 2023 Feb 6. Immunol Invest. 2023. PMID: 36745138 Review. - Gegen Qinlian decoction ameliorates TNBS-induced ulcerative colitis by regulating Th2/Th1 and Tregs/Th17 cells balance, inhibiting NLRP3 inflammasome activation and reshaping gut microbiota.
Hu Y, Tang J, Xie Y, Xu W, Zhu W, Xia L, Fang J, Yu D, Liu J, Zheng Z, Zhou Q, Shou Q, Zhang W. Hu Y, et al. J Ethnopharmacol. 2024 Jun 28;328:117956. doi: 10.1016/j.jep.2024.117956. Epub 2024 Feb 29. J Ethnopharmacol. 2024. PMID: 38428658 - NLRP3 Inflammasome and Inflammatory Bowel Disease.
Zhen Y, Zhang H. Zhen Y, et al. Front Immunol. 2019 Feb 28;10:276. doi: 10.3389/fimmu.2019.00276. eCollection 2019. Front Immunol. 2019. PMID: 30873162 Free PMC article. Review.
Cited by
- The Antidepressant Mirtazapine Rapidly Shifts Hepatic B Cell Populations and Functional Cytokine Signatures in the Mouse.
Almishri W, Davis RP, Shaheen AA, Altonsy MO, Jenne CN, Swain MG. Almishri W, et al. Front Immunol. 2021 Mar 25;12:622537. doi: 10.3389/fimmu.2021.622537. eCollection 2021. Front Immunol. 2021. PMID: 33841403 Free PMC article. - NLRP6 in infection and inflammation.
Anand PK, Kanneganti TD. Anand PK, et al. Microbes Infect. 2013 Sep-Oct;15(10-11):661-8. doi: 10.1016/j.micinf.2013.06.009. Epub 2013 Jun 28. Microbes Infect. 2013. PMID: 23811097 Free PMC article. Review. - Protective and detrimental roles of inflammasomes in disease.
Saavedra PH, Demon D, Van Gorp H, Lamkanfi M. Saavedra PH, et al. Semin Immunopathol. 2015 Jul;37(4):313-22. doi: 10.1007/s00281-015-0485-5. Epub 2015 Apr 21. Semin Immunopathol. 2015. PMID: 25895577 Review. - Inflammatory Responses of Porcine MoDC and Intestinal Epithelial Cells in a Direct-Contact Co-culture System Following a Bacterial Challenge.
Loss H, Aschenbach JR, Ebner F, Tedin K, Lodemann U. Loss H, et al. Inflammation. 2020 Apr;43(2):552-567. doi: 10.1007/s10753-019-01137-4. Inflammation. 2020. PMID: 31811548 - Does seasonality of the microbiota contribute to the seasonality of acute gout flare?
Schlesinger N, Brunetti L, Androulakis IP. Schlesinger N, et al. Clin Exp Rheumatol. 2022 Sep;40(9):1793-1800. doi: 10.55563/clinexprheumatol/hdtge7. Epub 2022 Mar 30. Clin Exp Rheumatol. 2022. PMID: 35383564 Free PMC article. Review.
References
- Xavier RJ, Podolsky DK. Unravelling the pathogenesis of inflammatory bowel disease. Nature. 2007;448:427–434. - PubMed
- Netea MG, Ferwerda G, de Jong DJ, et al. NOD2 30-0insC mutation and the pathogenesis of Crohn’s disease: impaired IL-1beta production points to a loss-of-function phenotype. Neth J Med. 2005;63:305–308. - PubMed
- Li J, Moran T, Swanson E, et al. Regulation of IL-8 and IL-1beta expression in Crohn’s disease associated NOD2/CARD15 mutations. Hum Mol Genet. 2004;13:1715–1725. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- P30 DK040561/DK/NIDDK NIH HHS/United States
- R01 DK060049/DK/NIDDK NIH HHS/United States
- CAPMC/ CIHR/Canada
- R01 DK064869/DK/NIDDK NIH HHS/United States
- P01 DE013499/DE/NIDCR NIH HHS/United States
- DE13499/DE/NIDCR NIH HHS/United States
- R29 DK050189/DK/NIDDK NIH HHS/United States
- HL60569/HL/NHLBI NIH HHS/United States
- R01 HL060569/HL/NHLBI NIH HHS/United States
- R01 DK050189/DK/NIDDK NIH HHS/United States
- R37 DK050189/DK/NIDDK NIH HHS/United States
- DK50189/DK/NIDDK NIH HHS/United States
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous