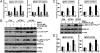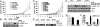p21-activated kinase 4 regulates ovarian cancer cell proliferation, migration, and invasion and contributes to poor prognosis in patients - PubMed (original) (raw)
. 2010 Oct 26;107(43):18622-7.
doi: 10.1073/pnas.0907481107. Epub 2010 Oct 6.
Hoi Yan Chan, Daniel S H Kong, Esther S Y Wong, Oscar G W Wong, Hextan Y S Ngan, Kar Fai Tam, Hongquan Zhang, Zhilun Li, Queeny K Y Chan, Sai Wah Tsao, Staffan Strömblad, Annie N Y Cheung
Affiliations
- PMID: 20926745
- PMCID: PMC2972956
- DOI: 10.1073/pnas.0907481107
p21-activated kinase 4 regulates ovarian cancer cell proliferation, migration, and invasion and contributes to poor prognosis in patients
Michelle K Y Siu et al. Proc Natl Acad Sci U S A. 2010.
Abstract
Ovarian cancer is a lethal gynecological malignancy, and to improve survival, it is important to identify novel prognostic and therapeutic targets. In this study, we present a role for p21-activated kinase 4 (Pak4) in ovarian cancer progression. We show a significant association between increased expression of Pak4 and its activated form, phosphorylated (p)-Pak4 Ser(474), with metastasis of ovarian cancers, shorter overall and disease-free survival, advanced stage and high-grade cancers, serous/clear cell histological subtypes, and reduced chemosensitivity. Pak4 overexpression was also observed in ovarian cancer cell lines. Pak4 and p-Pak4 expression were detected both in the nucleus and cytoplasm of ovarian cancer cells, in vitro as well as in vivo. Stable knockdown of Pak4 in ovarian cancer cell lines led to reduced cell migration, invasion, and proliferation, along with reduced c-Src, ERK1/2, and epidermal growth factor receptor (EGFR) activation and decreased matrix metalloproteinase 2 (MMP2) expression. Conversely, Pak4 overexpression promoted ovarian cancer cell migration and invasion in a c-Src, MEK-1, MMP2, and kinase-dependent manner, and induced cell proliferation through the Pak4/c-Src/EGFR pathway that controls cyclin D1 and CDC25A expression. Stable knockdown of Pak4 also impeded tumor growth and dissemination in nude mice. This report reveals the association between Pak4 and important clinicopathologic parameters, suggesting Pak4 to be a significant prognostic marker and potential therapeutic molecular target in ovarian cancer. The implied possible cross-talk between Pak4 and EGFR suggests the potential of dual targeting of EGFR and Pak4 as a unique therapeutic approach for cancer therapy.
Conflict of interest statement
The authors declare no conflict of interest.
Figures
Fig. 1.
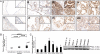
Overexpression of Pak4 and p-Pak4 (the activated form) in ovarian cancer. (A) Immunohistochemical staining of p-Pak4 in serous (i) and mucinous (v) benign ovarian cystadenomas, serous (ii) and mucinous (vi) borderline ovarian tumors and serous (iii), mucinous (vii), clear-cell (iv), and endometrioid (viii) ovarian carcinomas. (Insets) Regions with higher magnification. (B) qPCR analysis of Pak4 mRNA in ovarian tumors. The fold change of Pak4 mRNA was calculated with respect to the lowest expression of Pak4 in cystadenomas. (C) mRNA (Left) and protein (Right) expression of Pak4 in immortalized ovarian epithelial cell lines and ovarian cancer cell lines as determined by qPCR (*P < 0.05 compared with HOSE 11-12) and immunoblot analysis, respectively.
Fig. 2.
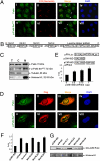
Localization of Pak4 and p-Pak4 in the nucleus and cytoplasm of ovarian cancer cells and activation of gene transcription by Pak4. (A) Subcellular localization of Pak4 and p-Pak4 in OVCAR-3 determined by confocal microscope. Cells were stained with rabbit anti-Pak4 antibody (i) or rabbit anti-p-Pak4 antibody (v) along with mouse anti C23-nucleolin antibody (ii and vi). Merge images displays Pak4 colocalization with C23-nucleolin (iii). p-Pak4 was not colocalized with C23-nucleolin in merge image (vii). White arrowheads indicated the cytoplasmic localization of Pak4 and p-Pak4. (iv and viii) DAPI staining of nuclei. (B) Schematic drawing illustrating four possible nuclear localization signals (NLSs 1–4) and a leucine zipper pattern and their positions on Pak4 protein. (C) Pak4 and p-Pak4 in subcellular protein fractions (T, total cell lysate; C, cytoplasmic fraction; N, nuclear fraction) of OVCAR-3. (D) Immunofluorescent staining of Flag-tagged wt Pak4 overexpressing SKOV-3 cells by rabbit anti-Pak4 antibody (i) or rabbit anti–p-Pak4 antibody (v) along with mouse anti-Flag tag antibody (ii and vi). Exogenous wt Pak4 was detected in the nucleus and cytoplasm of SKOV-3 cells and colocalized with fluorescent staining as detected by anti-Pak4 antibody (iii) or rabbit anti–p-Pak4 antibody (vii). (iv and viii) DAPI staining of nuclei. (E Upper) Schematic illustration of GAL4-Luc reporter plasmid, GAL4-BD vector (control), and GAL4-BD-wt Pak4 construct. (Lower) The GAL4-Luc activity of the reporter gene as fold of control; n = 3; *P < 0.05. (F) The GAL4-Luc activity in SKOV-3 cells expressing wt Pak4 or four NLS Pak4 mutants as fold of control; n = 3. (G) Immunoblot analyses of exogenous Pak4 in nuclear fractions extracted from SKOV-3 cells expressing wt Pak4 or Pak4 NLS mutants using anti-GAL4 DNA-BD antibody.
Fig. 3.
Pak4 abrogation inhibited ovarian cancer cell migration and invasion and reduced c-Src and ERK1/2 activation as well as MMP2 expression. (A) Stable knockdown of Pak4 mRNA and protein in OVCAR-3 and OVCA420 as detected by qPCR (Upper) and immunoblot analysis (Lower), respectively. (B) Wound-healing assay in control and transient knockdown of Pak4 in OVCA420. In vitro migration (C) and invasion assays (D) using Transwell membrane without or with Matrigel coating, respectively. (Upper) Representative images of migrating or invading OVCA420 cells. (Lower) Cell migration or invasion from OVCAR-3 and OVCA420 presented as percentage of control; n = 3; **P < 0.005. (E) Immunoblot analysis on FAK, c-Src, ERK1/2, p38, MMP2, and EGFR expression and/or activation in control and shPak4 OVCA420. (F) Immunoblot analysis of MMP2 expression using conditioned media prepared from control and shPak4 OVCA420.
Fig. 4.
Pak4-mediated cell migration and invasion in ovarian cancer cells involved c-Src and MEK-1/ERK1/2/MMP2 pathways. (A, C, and D) In vitro migration (Left) and invasion (Right) assays in SKOV-3 cells stably transfected with Flag-tagged wt Pak4, ca Pak4, or control vector in the presence or absence of PP1 (c-Src inhibitor), U0126 (MEK-1 inhibitor), PD98059 (MEK-1 inhibitor), OA-Hy (MMP2 inhibitor), or DMSO (vehicle). Cell migration or invasion presented as percentage of control; n = 3; *P < 0.05, **P < 0.005. (B) Immunoblot analyses of exogenous Flag-tagged Pak4 and MMP2 levels; and c-Src and ERK1/2 activities in SKOV-3 cells expressing wt or ca Pak4 in the presence or absence of PP1 or U0126. (C, Lower) Immunoblot analysis of ERK1/2 activation in wt or ca Pak4 overexpressing SKOV-3 cells in the presence or absence of PD98059.
Fig. 5.
Pak4-induced proliferation involved the c-Src/EGFR pathway that controls cyclin D1 and CDC25A expression. (A and C) Cell proliferation rate of wt or ca Pak4 overexpressing SKOV-3 cells in the presence or absence of PP1, CL387, 785 or PD153035. (E) Cell proliferation rate of OVCA420 in control and shPak4 after 12 d displayed as fold change compared with control; n = 3; **P < 0.005. (B and D) Immunoblot analysis of p-EGFR Tyr845 and EGFR expression in SKOV-3 cells overexpressing wt or ca Pak4 in the presence or absence of PP1, CL or PD153035. (F) mRNA expression of cyclin D1 and CDC25A in ca Pak4 overexpressing cells displayed as percentage of control (Flag vector-transfected SKOV-3 cells with DMSO) in the presence or absence of CL by qPCR; n = 3; *P < 0.05. (G) Immunoblot analysis of cyclin D1 expression in ca Pak4 overexpressing cells in the presence or absence of PP1 or CL.
Fig. 6.
Pak4 depletion impeded tumor growth and dissemination in nude mice. (A) Growth rates of s.c. tumors formed in mice inoculated with shPak4 ES-2 cells or control cells (2 × 106). (B) Representative views of the abdominal cavity of mice inoculated i.p. with shPak4 ES-2 cells or control cells. Arrows, tumors.
Similar articles
- LCH-7749944, a novel and potent p21-activated kinase 4 inhibitor, suppresses proliferation and invasion in human gastric cancer cells.
Zhang J, Wang J, Guo Q, Wang Y, Zhou Y, Peng H, Cheng M, Zhao D, Li F. Zhang J, et al. Cancer Lett. 2012 Apr 1;317(1):24-32. doi: 10.1016/j.canlet.2011.11.007. Epub 2011 Nov 13. Cancer Lett. 2012. PMID: 22085492 - Overexpression of P21-activated kinase 4 is associated with poor prognosis in non-small cell lung cancer and promotes migration and invasion.
Cai S, Ye Z, Wang X, Pan Y, Weng Y, Lao S, Wei H, Li L. Cai S, et al. J Exp Clin Cancer Res. 2015 May 15;34(1):48. doi: 10.1186/s13046-015-0165-2. J Exp Clin Cancer Res. 2015. PMID: 25975262 Free PMC article. - Activated-PAK4 predicts worse prognosis in breast cancer and promotes tumorigenesis through activation of PI3K/AKT signaling.
He LF, Xu HW, Chen M, Xian ZR, Wen XF, Chen MN, Du CW, Huang WH, Wu JD, Zhang GJ. He LF, et al. Oncotarget. 2017 Mar 14;8(11):17573-17585. doi: 10.18632/oncotarget.7466. Oncotarget. 2017. PMID: 28407679 Free PMC article. - PAK4 signaling in health and disease: defining the PAK4-CREB axis.
Won SY, Park JJ, Shin EY, Kim EG. Won SY, et al. Exp Mol Med. 2019 Feb 12;51(2):1-9. doi: 10.1038/s12276-018-0204-0. Exp Mol Med. 2019. PMID: 30755582 Free PMC article. Review. - PAK4 in cancer development: Emerging player and therapeutic opportunities.
Yuan Y, Zhang H, Li D, Li Y, Lin F, Wang Y, Song H, Liu X, Li F, Zhang J. Yuan Y, et al. Cancer Lett. 2022 Oct 1;545:215813. doi: 10.1016/j.canlet.2022.215813. Epub 2022 Jul 4. Cancer Lett. 2022. PMID: 35798086 Review.
Cited by
- Targeting PAK4 reverses cisplatin resistance in NSCLC by modulating ER stress.
Liu S, Yang P, Wang L, Zou X, Zhang D, Chen W, Hu C, Xiao D, Ren H, Zhang H, Cai S. Liu S, et al. Cell Death Discov. 2024 Jan 18;10(1):36. doi: 10.1038/s41420-024-01798-7. Cell Death Discov. 2024. PMID: 38238316 Free PMC article. - Group II p21-activated kinases as therapeutic targets in gastrointestinal cancer.
Shao YG, Ning K, Li F. Shao YG, et al. World J Gastroenterol. 2016 Jan 21;22(3):1224-35. doi: 10.3748/wjg.v22.i3.1224. World J Gastroenterol. 2016. PMID: 26811660 Free PMC article. Review. - p21-Activated Kinase 4 Signaling Promotes Japanese Encephalitis Virus-Mediated Inflammation in Astrocytes.
He W, Zhao Z, Anees A, Li Y, Ashraf U, Chen Z, Song Y, Chen H, Cao S, Ye J. He W, et al. Front Cell Infect Microbiol. 2017 Jun 21;7:271. doi: 10.3389/fcimb.2017.00271. eCollection 2017. Front Cell Infect Microbiol. 2017. PMID: 28680855 Free PMC article. - PAK4 kinase activity and somatic mutation promote carcinoma cell motility and influence inhibitor sensitivity.
Whale AD, Dart A, Holt M, Jones GE, Wells CM. Whale AD, et al. Oncogene. 2013 Apr 18;32(16):2114-20. doi: 10.1038/onc.2012.233. Epub 2012 Jun 11. Oncogene. 2013. PMID: 22689056 Free PMC article. - Mbt/PAK4 together with SRC modulates N-Cadherin adherens junctions in the developing Drosophila eye.
Pütz SM. Pütz SM. Biol Open. 2019 Mar 18;8(3):bio038406. doi: 10.1242/bio.038406. Biol Open. 2019. PMID: 30885947 Free PMC article.
References
- Jemal A, et al. Cancer statistics, 2007. CA Cancer J Clin. 2007;57:43–66. - PubMed
- Marugame T, Hirabayashi Y. Comparison of time trends in ovary cancer incidence (1973-1997) in East Asia, Europe, and the USA, from Cancer Incidence in Five Continents Vols IV VIII. Jpn J Clin Oncol. 2007;37:802–803. - PubMed
- Agarwal R, Kaye SB. Ovarian cancer: Strategies for overcoming resistance to chemotherapy. Nat Rev Cancer. 2003;3:502–516. - PubMed
- Kumar R, Gururaj AE, Barnes CJ. p21-activated kinases in cancer. Nat Rev Cancer. 2006;6:459–471. - PubMed
- Siu MK, et al. Differential expression and phosphorylation of Pak1 and Pak2 in ovarian cancer: Effects on prognosis and cell invasion. Int J Cancer. 2009;127:21–31. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
Research Materials
Miscellaneous