Colonic motor dysfunction in human diabetes is associated with enteric neuronal loss and increased oxidative stress - PubMed (original) (raw)
Colonic motor dysfunction in human diabetes is associated with enteric neuronal loss and increased oxidative stress
B Chandrasekharan et al. Neurogastroenterol Motil. 2011 Feb.
Abstract
Background: Gastrointestinal dysfunction is very common in diabetic patients. We assessed the changes in the colonic enteric nervous system using colectomy specimens and intestinal biopsies from diabetic subjects and age-matched controls.
Methods: In control and diabetic colons, we determined the total ganglion area (hematoxylin-eosin staining), changes in neuronal markers-protein gene product 9.5, peripherin, neuronal nitric oxide synthase (nNOS), neuropeptide Y (NPY), choline acetyl transferase (ChAT) and vasoactive intestinal peptide (by immunostaining), apoptosis (cleaved caspase-3 staining) and reduced glutathione levels. Superoxide dismutase mRNA was determined in enteric ganglia isolated by laser capture micro dissection. Isometric muscle recording was used to assess contraction and relaxation responses of colonic circular muscle strips. Apoptosis in enteric neurons under hyperglycemia in vitro was determined by cleaved caspase-3 Western blotting and protective effects of lipoic acid were evaluated.
Key results: Diabetic subjects had higher incidence of lower gastrointestinal symptoms like constipation and diarrhea at baseline prior to surgery. Diabetic ganglia displayed significant decrease in ganglion size due to enhanced apoptosis and loss of peripherin, nNOS, NPY, and ChAT neurons. Reduced glutathione levels in the diabetic colon (HbA1C > 7%) were significantly less than the control, indicating increased oxidative stress. Colonic circular muscle strips from diabetic subjects showed impaired contraction and relaxation responses compared with the healthy controls. Hyperglycemia-induced cleaved caspase-3 in enteric neurons was reversed by lipoic acid.
Conclusions & inferences: Our data demonstrate loss of enteric neurons in the colon due to increased oxidative stress and apoptosis which may cause the motility disturbances seen in human diabetes. Antioxidants may be of therapeutic value for preventing motility disorders in diabetes.
© 2010 Blackwell Publishing Ltd.
Figures
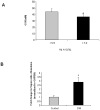
Figure 1. Diabetic colon exhibits decreased ganglion size and increased apoptosis
The colon from diabetic patients (DM) and controls were embedded in paraffin and 5 μm sections were stained using hematoxylin and eosin (H/E), antibodies for PGP 9.5, Peripherin and cleaved caspase-3. Total area as well as the percent of neuronal ganglion area stained for a specific neuronal marker was measured using calibration and measurement options available in the Metamorph software. Cleaved caspase-3 positive cells were counted and expressed as percentage of total peripherin positive cells in the ganglion. (A) There was a decrease in the ganglion size in diabetic colon, (B) Peripherin and PGP 9.5 area was decreased in diabetic colon and (C) Percent Cleaved caspase-3 neurons was increased in diabetic colon. Values are the mean ± SEM, n = 8, * P < 0.05, ** P < 0.01, Magnification 40 X.
Figure 1. Diabetic colon exhibits decreased ganglion size and increased apoptosis
The colon from diabetic patients (DM) and controls were embedded in paraffin and 5 μm sections were stained using hematoxylin and eosin (H/E), antibodies for PGP 9.5, Peripherin and cleaved caspase-3. Total area as well as the percent of neuronal ganglion area stained for a specific neuronal marker was measured using calibration and measurement options available in the Metamorph software. Cleaved caspase-3 positive cells were counted and expressed as percentage of total peripherin positive cells in the ganglion. (A) There was a decrease in the ganglion size in diabetic colon, (B) Peripherin and PGP 9.5 area was decreased in diabetic colon and (C) Percent Cleaved caspase-3 neurons was increased in diabetic colon. Values are the mean ± SEM, n = 8, * P < 0.05, ** P < 0.01, Magnification 40 X.
Figure 2. Enteric neuronal subpopulations are altered in diabetes
The colon from diabetic patients (DM) and controls (Con) were embedded in paraffin and 5 μm sections were immunostained for nNOS, NPY, ChAT and VIP neurons as detailed in Methods section. The total area and percentage of ganglion area stained for the respective neuronal marker was assessed by Metamorph software. (A) Both the total area and per cent area stained were decreased for nNOS in diabetic (DM) colon, (B) Per cent of ganglion area stained for NPY was decreased in the DM colon, (C) Total ChAT area (but not per cent ChAT-stained ganglion area) was decreased in DM, however VIP area and % of ganglion area was unchanged in DM (D). Values are the mean ± SEM, n = 8, ** P< 0.01, *** P<0.001.
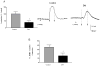
Figure 3. Decreased Glutathione (GSH) and increased SOD expression in colonic ganglia from diabetic patients
The colon from diabetic (n =6) and control (n =14) subjects were flash-frozen and assayed for reduced glutathione (GSH) by HPLC. The enteric ganglia were isolated by laser capture micro dissection and RNA obtained was amplified for superoxide dismutase (SOD) expression by real time PCR. (A) Reduced Glutathione (GSH) levels were decreased in the DM colon and (B) Increased SOD mRNA (normalized to GAPDH) in the ganglia of diabetic colon compared to control (n = 3). The values are the mean ± SEM, * P < 0.05.
Figure 4. Circular muscle strips from the DM colon exhibit significantly less contraction and relaxation
The circular muscle strips from the colon of diabetic and control subjects were allowed to equilibrate for 2 h and pre-treated with L-NAME (nitric oxide inhibitor) for 20 min before electrical field stimulation (EFS- 20 Hz, 0.5 ms) to study the contractile responses. The strips were washed and then pre-treated with atropine and guanethidine sulfate for 20 min before EFS (4 Hz, 0.3 ms) to assess relaxation. (A) EFS-induced contraction responses in the DM colon expressed as percent of response in the control colon (n= 9) (B) Change in contraction in the presence of L-NAME in the DM and the control colon is shown (n = 6) and (C) Relaxation responses in the DM colon expressed as percent of response in the control colon (n = 8). Representative contraction and relaxation tracings in control and diabetic colonic tissue are given; force is represented in milli newtons (mN) and time in seconds or min. The arrows represent ‘on’ and ‘off’ of electrical current. Results are mean ± SEM, * P < 0.05.
Figure 4. Circular muscle strips from the DM colon exhibit significantly less contraction and relaxation
The circular muscle strips from the colon of diabetic and control subjects were allowed to equilibrate for 2 h and pre-treated with L-NAME (nitric oxide inhibitor) for 20 min before electrical field stimulation (EFS- 20 Hz, 0.5 ms) to study the contractile responses. The strips were washed and then pre-treated with atropine and guanethidine sulfate for 20 min before EFS (4 Hz, 0.3 ms) to assess relaxation. (A) EFS-induced contraction responses in the DM colon expressed as percent of response in the control colon (n= 9) (B) Change in contraction in the presence of L-NAME in the DM and the control colon is shown (n = 6) and (C) Relaxation responses in the DM colon expressed as percent of response in the control colon (n = 8). Representative contraction and relaxation tracings in control and diabetic colonic tissue are given; force is represented in milli newtons (mN) and time in seconds or min. The arrows represent ‘on’ and ‘off’ of electrical current. Results are mean ± SEM, * P < 0.05.
Figure 5. Hyperglycemia induces increased apoptosis and reduction in phosphatidyl inositol-3-kinase (P-I-3-K) signaling in enteric neurons which is reversed by the antioxidant lipoic acid
Enteric neuronal murine cell lines were treated with 5 and 40 mM glucose in the presence or absence of lipoic acid (10 μM). After 48 h, cell lysates were collected and probed for cleaved caspase-3 and phospho-Akt (pAkt) by Western blotting. β-actin was used as the loading control. (A) Increased cleaved caspase-3 and reduced phospho-Akt (pAkt) under hyperglycemia in enteric neurons, the results from densitometric analyses of the bands using Scion Image are graphically represented in (B) and (C). Results are mean ± SEM, n = 4, *** P<0.001, * P < 0.05.
Comment in
- Oxidative stress: key player in gastrointestinal complications of diabetes.
Kashyap P, Farrugia G. Kashyap P, et al. Neurogastroenterol Motil. 2011 Feb;23(2):111-4. doi: 10.1111/j.1365-2982.2010.01659.x. Neurogastroenterol Motil. 2011. PMID: 21226884 Free PMC article.
References
- Lomax AE, Sharkey KA, Furness JB. The participation of the sympathetic innervation of the gastrointestinal tract in disease states. Neurogastroenterol Motil. 2010;22:7–18. - PubMed
- Bytzer P, Talley NJ, Leemon M, Young LJ, Jones MP, Horowitz M. Prevalence of gastrointestinal symptoms associated with diabetes mellitus: a population-based survey of 15,000 adults. Arch Intern Med. 2001;161:1989–1996. - PubMed
- Samsom M, Vermeijden JR, Smout AJ, et al. Prevalence of delayed gastric emptying in diabetic patients and relationship to dyspeptic symptoms: a prospective study in unselected diabetic patients. Diabetes Care. 2003;26:3116–3122. - PubMed
- Feldman M, Schiller LR. Disorders of gastrointestinal motility associated with diabetes mellitus. Ann Intern Med. 1983;98:378–384. - PubMed
- Camilleri M. Gastrointestinal problems in diabetes. Endocrinol Metab Clin North Am. 1996;25:361–378. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- R01 DK06411/DK/NIDDK NIH HHS/United States
- R03 DK078552/DK/NIDDK NIH HHS/United States
- K08 DK067045/DK/NIDDK NIH HHS/United States
- R24 DK064399/DK/NIDDK NIH HHS/United States
- DK064399/DK/NIDDK NIH HHS/United States
- R01 DK080684/DK/NIDDK NIH HHS/United States
- R01 DK064711/DK/NIDDK NIH HHS/United States
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
Miscellaneous