Akt and autophagy cooperate to promote survival of drug-resistant glioma - PubMed (original) (raw)
. 2010 Nov 9;3(147):ra81.
doi: 10.1126/scisignal.2001017.
Christine Cheng, Chris Hackett, Morri Feldman, Benjamin T Houseman, Theodore Nicolaides, Daphne Haas-Kogan, C David James, Scott A Oakes, Jayanta Debnath, Kevan M Shokat, William A Weiss
Affiliations
- PMID: 21062993
- PMCID: PMC3001107
- DOI: 10.1126/scisignal.2001017
Akt and autophagy cooperate to promote survival of drug-resistant glioma
Qi-Wen Fan et al. Sci Signal. 2010.
Abstract
Although the phosphatidylinositol 3-kinase to Akt to mammalian target of rapamycin (PI3K-Akt-mTOR) pathway promotes survival signaling, inhibitors of PI3K and mTOR induce minimal cell death in PTEN (phosphatase and tensin homolog deleted from chromosome 10) mutant glioma. Here, we show that the dual PI3K-mTOR inhibitor PI-103 induces autophagy in a form of glioma that is resistant to therapy. Inhibitors of autophagosome maturation cooperated with PI-103 to induce apoptosis through the mitochondrial pathway, indicating that the cellular self-digestion process of autophagy acted as a survival signal in this setting. Not all inhibitors of mTOR synergized with inhibitors of autophagy. Rapamycin delivered alone induced autophagy, yet cells survived inhibition of autophagosome maturation because of rapamycin-mediated activation of Akt. In contrast, adenosine 5'-triphosphate-competitive inhibitors of mTOR stimulated autophagy more potently than did rapamycin, with inhibition of mTOR complexes 1 and 2 contributing independently to induction of autophagy. We show that combined inhibition of PI3K and mTOR, which activates autophagy without activating Akt, cooperated with inhibition of autophagy to cause glioma cells to undergo apoptosis. Moreover, the PI3K-mTOR inhibitor NVP-BEZ235, which is in clinical use, synergized with the lysosomotropic inhibitor of autophagy, chloroquine, another agent in clinical use, to induce apoptosis in glioma xenografts in vivo, providing a therapeutic approach potentially translatable to humans.
Conflict of interest statement
Competing interests: The authors declare that they have no competing interests.
Figures
Fig. 1
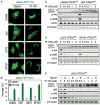
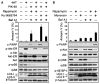
The dual PI3K and mTOR inhibitor PI-103 induces autophagy in PTENwt and PTENmt glioma cell lines. (A) PTENwt (LN229), PTENwt (SF767), PTENmt (U87), and PTENmt (U373) cell lines were transduced with pBabe-GFP-LC3 and treated with PI-103 (1 μM) for 48 hours. PI-103–treated cells show punctate patterns of GFP staining, typical of autophagy. (B) Quantification of punctate foci from (A). The percentage of cells accumulating LC3-GFP in vacuoles was quantified as the mean ± SD (five high-power microscopic fields were counted for each value). (C and D) Glioma cells wild type (LN229, SF767) or mutant (U87, U373) for PTEN were treated with PI-103 at concentrations shown, lysed, and analyzed by immunoblot. PI-103 decreased phosphorylation of Akt and that of the mTOR target p-rpS6 in all cell lines. Activation of autophagy was dose-dependent, as indicated by increased amounts of the autophagy marker LC3-II. (E) U373 cells were treated with PI-103 at concentrations shown for 48 hours, treated with 10 nM Baf A1 for 2 hours, lysed, and analyzed by immunoblot for p62, an LC3-binding protein and marker of autophagic flux. Degradation of p62 indicates induction of autophagy. β-Tubulin shown as a loading control.
Fig. 2
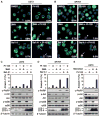
PI-103 synergizes with Baf A1, an inhibitor of autophagosome maturation, to induce apoptosis in glioma. Glioma cells (U373, PTEN mt and SF767, PTEN wt) were transduced with pBabe-GFP-LC3, treated with DMSO or PI-103 (1 μM) for 24 hours, and then treated for 48 hours with either 3MA (5 mM) or Baf A1 (10 nM). (A and B) Cytospin preparations were stained with antibody against cleaved caspase 3 (red) or GFP-LC3 (green). Nuclei stained in blue (Hoechst dye). Arrowheads indicate cleaved caspase 3–positive cells, with percentages indicated. Arrows show cleaved caspase 3–positive cells with condensed GFP-LC3 punctate dots. Scale bar, 100 μm. (C and D) Apoptotic cells were analyzed by flow cytometry. Percentages of cells positive for annexin V are the mean ± SE for triplicate samples (U373: P < 0.0001 by Student’s t test for PI-103 plus 3MA versus DMSO; P < 0.0001 for PI-103 plus Baf A1 versus DMSO; SF767: P < 0.0001 by Student’s t test for PI-103 plus 3MA versus DMSO; P < 0.0001 for PI-103 plus Baf A1 versus DMSO). Lysed cells were analyzed by immunoblot with antisera indicated. (E) To exclude off-target effects of Baf A1 independent of lysosomal trafficking, we treated U373 PTEN mt glioma cells with 1 μM PI-103 or DMSO for 24 hours. Monensin (3 μM) was added where indicated (24 hours), and cells were analyzed by flow cytometry for annexin V. Data show error among triplicate measurements for each value (top panel). An aliquot of cells was analyzed by immunoblot with antisera indicated (bottom panel).
Fig. 3
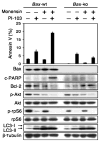
Apoptosis induced by combined inhibition of PI3K and mTOR signaling and of lysosomal trafficking proceeds through the intrinsic mitochondrial pathway. MEFs wild type for Bax show modest apoptosis in response to PI-103 (1 μM) but not to monensin (3 μM). Combination therapy with monensin and PI-103 led to apoptosis only in cells wild type for Bax, demonstrating that apoptosis induced by monensin and PI-103 depends on the mitochondrial pathway. Data show error among triplicate measurements for each value (top panel). An aliquot of cells was lysed and analyzed by immunoblot with antisera indicated (bottom panel). A blot representative of two independent experiments is shown.
Fig. 4
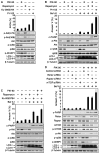
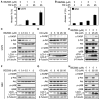
Combined inhibition of mTOR and PI3K signaling and of autophagosome maturation leads to apoptosis in U373 PTEN mt glioma. (A) The mTOR kinase inhibitor Ku-0063794 induced autophagy more potently than did the allosteric mTORC1 inhibitor rapamycin or the PI3Kα inhibitor PIK-90. Cells were treated with DMSO, PIK-90 (1 μM), rapamycin (100 nM), Ku-0063794 (5 μM), or PI-103 (1 μM) for 48 hours and stained with acridine orange (1 μg/ml) for 15 min. Acidic vesicular organelles were quantified (top panel). Cells treated for 24 hours were examined by immunoblot with antisera shown (bottom panel). (B) Apoptosis induced by Baf A1 requires concurrent inhibition of PI3K and mTOR. U373 glioma cells were treated with PIK-90 (1 μM), rapamycin (100 nM), PIK-90 plus rapamycin, or PI-103 (1 μM) for 24 hours. Baf A1 (10 nM) was added for 48 hours, and cells were analyzed by flow cytometry for annexin V and by immunoblot. (C) Blockade of autophagosome maturation induces apoptosis when combined with Ku-0063794, or with rapamycin and PIK-90 in combination. U373 glioma cells were treated with 1 μM PIK-90, 5 μM Ku-0063794, 1 μM PIK-90 plus 100 nM rapamycin, or 1 μM PIK-90 plus 5 μM Ku-0063794 for 24 hours. Baf A1 (10 nM) was added for 48 hours, and cells were analyzed as in (B). (D) To probe a role for mTORC1 and mTORC2 in the induction of autophagy and in apoptosis, we transfected U373 glioma cells with control siRNA, or siRNA against rictor, raptor, or to mTOR itself (24 hours). Cells were subsequently treated with PIK-90 (1 μM) and Baf A1 (10 nM) for 48 hours and analyzed as in (B). A blot representative of two independent experiments is shown. Data shown are means ± SD for triplicate measurements in (A), (B), (C), and (D) (top panel).
Fig. 5
Activation of Akt cooperates with induction of autophagy to promote therapeutic resistance in glioma. (A) U373 glioma cells were transduced with Akt-ER, an allele of Akt fused to the steroid-binding domain of the ER (57 ). Cells were treated with PIK-90 (1 μM), rapamycin (100 nM), or Ku-0063794 (5 μM) for 24 hours. Baf A1 (10 nM) was added in the presence or absence of 4HT (500 nM) for 48 hours. Cells were analyzed by annexin V–FITC flow cytometry and by immunoblot. Activation of Akt-ER blocked apoptosis in cells treated with Baf A1, rapamycin, and PIK-90 or with Baf A1, Ku-0063794, and PIK-90. These data suggest that the induction of p-Akt serves as a survival signal, promoting resistance to apoptosis in glioma. (B) Monensin also blocks activation of Akt (Figs. 2E and 3). To address whether monensin could synergize with rapamycin to induce death, we treated U373 glioma cells with monensin (3 μM), rapamycin (100 nM), or both agents in combination (48 hours). Cells were analyzed by annexin V–FITC flow cytometry and by immunoblot. Monensin cooperated with rapamycin to induce cell death.
Fig. 6
The clinically used drugs BEZ235 and chloroquine cooperate to cause apoptosis in PTEN mt glioma cell lines. U373 PTENmt cells and cells derived from a PTEN mt xenograft of glioblastoma multiforme (GS2) were treated with the PI3K-mTOR inhibitor NVP-BEZ235 (BEZ) or the antimalarial autophagy and lysosomal trafficking inhibitor chloroquine (CQ) or both drugs. (A and B) BEZ and CQ cooperate to induce apoptosis (annexin V flow cytometry) in U373 and GS2 cells. Cells were treated with BEZ or vehicle (24 hours); CQ was then added where indicated (48 hours). (C to H) Dose response with single agents and effects of combination treatment in U373 and GS2 cells. The combination of BEZ and CQ induced apoptosis in both cell lines, whereas neither agent alone induced apoptosis (assessed by PARP cleavage). (C, D, F, and G) BEZ and CQ treatments were for 48 hours. (E and H) Treatments were as in (A) and (B).
Fig. 7
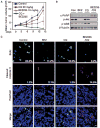
NVP-BEZ235 and chloroquine cause apoptosis and induce regression of established GS2 flank xenografts. (A) Animals with established tumors were treated by daily intraperitoneal injection as shown, n = 5 per group. BrdU was added 2 hours before animals were killed (day 16). Animals were killed 30 min after their last treatment with vehicle, chloroquine, BEZ, or combination therapy. (B and C) Residual tumors were analyzed by immunoblot (B) and by immunofluorescence staining for BrdU (a measure of proliferation) and cleaved caspase 3, a measure of apoptosis (C). Cells positive for BrdU and cleaved caspase 3 were counted in five high-power microscopic fields. Numbers denote the percentage of BrdU-and cleaved caspase 3–positive cells. Quantification of five high-power microscopic fields from five animals per group demonstrated an increase in cleaved caspase 3 levels from 1.2% (chloroquine monotherapy) to 14.8% (NVP-BEZ235 plus chloroquine; P < 0.0001, Student’s t test). The amount of apoptosis was similar in animals treated with monotherapy, 1.2% control versus 2.1% for NVP-BEZ235 monotherapy (P = 0.4793, Student’s t test), 1.2% control versus 1.2% for chloroquine monotherapy (P = 0.8822, Student’s t test). Scale bar, 100 μm.
Similar articles
- Inhibition of Autophagy Increases Proliferation Inhibition and Apoptosis Induced by the PI3K/mTOR Inhibitor NVP-BEZ235 in Breast Cancer Cells.
Ji Y, Di W, Yang Q, Lu Z, Cai W, Wu J. Ji Y, et al. Clin Lab. 2015;61(8):1043-51. doi: 10.7754/clin.lab.2015.150144. Clin Lab. 2015. PMID: 26427150 - Dual targeting of AKT and mammalian target of rapamycin: a potential therapeutic approach for malignant peripheral nerve sheath tumor.
Zou CY, Smith KD, Zhu QS, Liu J, McCutcheon IE, Slopis JM, Meric-Bernstam F, Peng Z, Bornmann WG, Mills GB, Lazar AJ, Pollock RE, Lev D. Zou CY, et al. Mol Cancer Ther. 2009 May;8(5):1157-68. doi: 10.1158/1535-7163.MCT-08-1008. Epub 2009 May 5. Mol Cancer Ther. 2009. PMID: 19417153 - Autophagy and Akt promote survival in glioma.
Fan QW, Weiss WA. Fan QW, et al. Autophagy. 2011 May;7(5):536-8. doi: 10.4161/auto.7.5.14779. Epub 2011 May 1. Autophagy. 2011. PMID: 21266843 Free PMC article. - Current development of the second generation of mTOR inhibitors as anticancer agents.
Zhou HY, Huang SL. Zhou HY, et al. Chin J Cancer. 2012 Jan;31(1):8-18. doi: 10.5732/cjc.011.10281. Epub 2011 Nov 4. Chin J Cancer. 2012. PMID: 22059905 Free PMC article. Review. - Xanthatin suppresses proliferation and tumorigenicity of glioma cells through autophagy inhibition via activation of the PI3K-Akt-mTOR pathway.
Chen H, Zhu T, Huang X, Xu W, Di Z, Ma Y, Xue M, Bi S, Shen Y, Yu Y, Shen Y, Feng L. Chen H, et al. Pharmacol Res Perspect. 2023 Feb;11(1):e01041. doi: 10.1002/prp2.1041. Pharmacol Res Perspect. 2023. PMID: 36572650 Free PMC article. Review.
Cited by
- Rapamycin-Loaded Lipid Nanocapsules Induce Selective Inhibition of the mTORC1-Signaling Pathway in Glioblastoma Cells.
Séhédic D, Roncali L, Djoudi A, Buchtova N, Avril S, Chérel M, Boury F, Lacoeuille F, Hindré F, Garcion E. Séhédic D, et al. Front Bioeng Biotechnol. 2021 Feb 25;8:602998. doi: 10.3389/fbioe.2020.602998. eCollection 2020. Front Bioeng Biotechnol. 2021. PMID: 33718332 Free PMC article. - Grape seed extract targets mitochondrial electron transport chain complex III and induces oxidative and metabolic stress leading to cytoprotective autophagy and apoptotic death in human head and neck cancer cells.
Shrotriya S, Deep G, Lopert P, Patel M, Agarwal R, Agarwal C. Shrotriya S, et al. Mol Carcinog. 2015 Dec;54(12):1734-47. doi: 10.1002/mc.22246. Epub 2014 Dec 31. Mol Carcinog. 2015. PMID: 25557495 Free PMC article. - Harnessing autophagy for adoptive T-cell therapy.
Amarnath S, Fowler DH. Amarnath S, et al. Immunotherapy. 2012 Jan;4(1):1-4. doi: 10.2217/imt.11.144. Immunotherapy. 2012. PMID: 22149992 Free PMC article. No abstract available. - Dual PI3K/mTOR inhibitor NVP-BEZ235 suppresses hypoxia-inducible factor (HIF)-1α expression by blocking protein translation and increases cell death under hypoxia.
Karar J, Cerniglia GJ, Lindsten T, Koumenis C, Maity A. Karar J, et al. Cancer Biol Ther. 2012 Sep;13(11):1102-11. doi: 10.4161/cbt.21144. Epub 2012 Aug 16. Cancer Biol Ther. 2012. PMID: 22895065 Free PMC article. - mir-30d Regulates multiple genes in the autophagy pathway and impairs autophagy process in human cancer cells.
Yang X, Zhong X, Tanyi JL, Shen J, Xu C, Gao P, Zheng TM, DeMichele A, Zhang L. Yang X, et al. Biochem Biophys Res Commun. 2013 Feb 15;431(3):617-22. doi: 10.1016/j.bbrc.2012.12.083. Epub 2012 Dec 27. Biochem Biophys Res Commun. 2013. PMID: 23274497 Free PMC article.
References
- Ma XM, Blenis J. Molecular mechanisms of mTOR-mediated translational control. Nat Rev Mol Cell Biol. 2009;10:307–318. - PubMed
- Kok K, Geering B, Vanhaesebroeck B. Regulation of phosphoinositide 3-kinase expression in health and disease. Trends Biochem Sci. 2009;34:115–127. - PubMed
- Parsons DW, Jones S, Zhang X, Lin JC, Leary RJ, Angenendt P, Mankoo P, Carter H, Siu IM, Gallia GL, Olivi A, McLendon R, Rasheed BA, Keir S, Nikolskaya T, Nikolsky Y, Busam DA, Tekleab H, Diaz LA, Jr, Hartigan J, Smith DR, Strausberg RL, Marie SK, Shinjo SM, Yan H, Riggins GJ, Bigner DD, Karchin R, Papadopoulos N, Parmigiani G, Vogelstein B, Velculescu VE, Kinzler KW. An integrated genomic analysis of human glioblastoma multiforme. Science. 2008;321:1807–1812. - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- R01 CA102321/CA/NCI NIH HHS/United States
- P50 CA097257-06S1/CA/NCI NIH HHS/United States
- R01 CA126792/CA/NCI NIH HHS/United States
- HHMI/Howard Hughes Medical Institute/United States
- P50 CA097257-09/CA/NCI NIH HHS/United States
- P50 CA097257-08/CA/NCI NIH HHS/United States
- P50CA097257/CA/NCI NIH HHS/United States
- P50 CA097257-07/CA/NCI NIH HHS/United States
- P50 CA097257-10/CA/NCI NIH HHS/United States
- R01 CA102321-08/CA/NCI NIH HHS/United States
- P50 CA097257-06/CA/NCI NIH HHS/United States
- P50 CA097257/CA/NCI NIH HHS/United States
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous