DNMT3A mutations in acute myeloid leukemia - PubMed (original) (raw)
. 2010 Dec 16;363(25):2424-33.
doi: 10.1056/NEJMoa1005143. Epub 2010 Nov 10.
Li Ding, Matthew J Walter, Michael D McLellan, Tamara Lamprecht, David E Larson, Cyriac Kandoth, Jacqueline E Payton, Jack Baty, John Welch, Christopher C Harris, Cheryl F Lichti, R Reid Townsend, Robert S Fulton, David J Dooling, Daniel C Koboldt, Heather Schmidt, Qunyuan Zhang, John R Osborne, Ling Lin, Michelle O'Laughlin, Joshua F McMichael, Kim D Delehaunty, Sean D McGrath, Lucinda A Fulton, Vincent J Magrini, Tammi L Vickery, Jasreet Hundal, Lisa L Cook, Joshua J Conyers, Gary W Swift, Jerry P Reed, Patricia A Alldredge, Todd Wylie, Jason Walker, Joelle Kalicki, Mark A Watson, Sharon Heath, William D Shannon, Nobish Varghese, Rakesh Nagarajan, Peter Westervelt, Michael H Tomasson, Daniel C Link, Timothy A Graubert, John F DiPersio, Elaine R Mardis, Richard K Wilson
Affiliations
- PMID: 21067377
- PMCID: PMC3201818
- DOI: 10.1056/NEJMoa1005143
DNMT3A mutations in acute myeloid leukemia
Timothy J Ley et al. N Engl J Med. 2010.
Abstract
Background: The genetic alterations responsible for an adverse outcome in most patients with acute myeloid leukemia (AML) are unknown.
Methods: Using massively parallel DNA sequencing, we identified a somatic mutation in DNMT3A, encoding a DNA methyltransferase, in the genome of cells from a patient with AML with a normal karyotype. We sequenced the exons of DNMT3A in 280 additional patients with de novo AML to define recurring mutations.
Results: A total of 62 of 281 patients (22.1%) had mutations in DNMT3A that were predicted to affect translation. We identified 18 different missense mutations, the most common of which was predicted to affect amino acid R882 (in 37 patients). We also identified six frameshift, six nonsense, and three splice-site mutations and a 1.5-Mbp deletion encompassing DNMT3A. These mutations were highly enriched in the group of patients with an intermediate-risk cytogenetic profile (56 of 166 patients, or 33.7%) but were absent in all 79 patients with a favorable-risk cytogenetic profile (P<0.001 for both comparisons). The median overall survival among patients with DNMT3A mutations was significantly shorter than that among patients without such mutations (12.3 months vs. 41.1 months, P<0.001). DNMT3A mutations were associated with adverse outcomes among patients with an intermediate-risk cytogenetic profile or FLT3 mutations, regardless of age, and were independently associated with a poor outcome in Cox proportional-hazards analysis.
Conclusions: DNMT3A mutations are highly recurrent in patients with de novo AML with an intermediate-risk cytogenetic profile and are independently associated with a poor outcome. (Funded by the National Institutes of Health and others.).
Figures
Figure 1. DNMT3A Mutations in 188 Patients with Acute Myeloid Leukemia (AML)
Shown are data from samples banked at Washington University that were obtained from 188 patients with AML. All mutations that are shown were confirmed to be somatic. The locations of the PWWP domain (characterized by the presence of a highly conserved proline–tryptophan–tryptophan–proline motif), the ADD (ATRX, DNMT3, and DNMT3L)-type zinc finger (ZNF) domain, and the methyltransferase (MTase) domain are shown. Each patient with a DNMT3A mutation is designated with a circle. The deletion that is noted at position 1 is a 1.5-Mbp deletion that includes DNMT3A and part or all of eight other genes (for details, see Fig. 2 in the Supplementary Appendix).
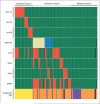
Figure 2. Relationship between DNMT3A Mutations and Other Common Mutations in 188 Patients with Acute Myeloid Leukemia (AML)
Shown is the mutation status of each of 188 patients with AML on the basis of sequencing results shown in Figure 1. Red indicates the presence of the specified mutation in the designated patient, and green the absence of the mutation. For DNMT3A mutations, the presence of any R882 variant is shown in tan, and all other DNMT3A mutations are shown in blue. DNMT3A, NPM1, and IDH1/2 mutations were not detected in any sample with t(15;17), t(8;21), complex t(8;21), or inv(16). In samples with DNMT3A mutations, mutations in FLT3 (either internal tandem duplications or mutations at amino acid position D835), NPM1, and IDH1 were significantly enriched. Cytogenetic risk groups are shown at the bottom of the graph, with yellow indicating favorable risk, orange intermediate risk, and purple adverse risk. Black boxes indicate that no data were available for that sample. Mutation groups as defined by these data are designated at the top of the graph. Samples in mutation group A have cytogenetic findings associated with favorable risk and have only FLT3 mutations among the five commonly mutated AML genes. Samples in mutation group B are highly enriched for mutations in the five commonly mutated AML genes that are associated with intermediate risk. Samples in mutation group C have no mutations in any of the five common AML genes, suggesting that a different set of mutations may contribute to the pathogenesis in this group.
Figure 3. Overall Survival among Patients with Acute Myeloid Leukemia (AML) with DNMT3A Mutations
Panel A shows the overall survival among all 281 patients, including 62 with a DNMT3A mutation and 219 without a DNMT3A mutation. Panel B shows the overall survival of 120 patients with normal karyotypes, including 44 with a DNMT3A mutation and 76 without a DNMT3A mutation. Panel C shows the overall survival of 54 patients with an FLT3 internal tandem duplication mutation, including 16 with a DNMT3A mutation and 38 without a DNMT3A mutation. Panel D shows the overall survival of all 281 patients stratified according to DNMT3A mutation status and age; 72 patients were over the age of 60 years and 209 were 60 years of age or younger. All P values, which are for the comparison between any DNMT3A mutation and no mutation, were calculated by means of the log-rank test.
Comment in
- Genetics, epigenetics, and leukemia.
Shannon K, Armstrong SA. Shannon K, et al. N Engl J Med. 2010 Dec 16;363(25):2460-1. doi: 10.1056/NEJMe1012071. Epub 2010 Nov 10. N Engl J Med. 2010. PMID: 21067376 Free PMC article. No abstract available.
Similar articles
- Persistence of DNMT3A R882 mutations during remission does not adversely affect outcomes of patients with acute myeloid leukaemia.
Bhatnagar B, Eisfeld AK, Nicolet D, Mrózek K, Blachly JS, Orwick S, Lucas DM, Kohlschmidt J, Blum W, Kolitz JE, Stone RM, Bloomfield CD, Byrd JC. Bhatnagar B, et al. Br J Haematol. 2016 Oct;175(2):226-236. doi: 10.1111/bjh.14254. Epub 2016 Aug 1. Br J Haematol. 2016. PMID: 27476855 Free PMC article. - DNMT3A (R882) mutation features and prognostic effect in acute myeloid leukemia in Coexistent with NPM1 and FLT3 mutations.
Kumar D, Mehta A, Panigrahi MK, Nath S, Saikia KK. Kumar D, et al. Hematol Oncol Stem Cell Ther. 2018 Jun;11(2):82-89. doi: 10.1016/j.hemonc.2017.09.004. Epub 2017 Oct 18. Hematol Oncol Stem Cell Ther. 2018. PMID: 29079128 Clinical Trial. - DNMT3A mutations in acute myeloid leukemia: stability during disease evolution and clinical implications.
Hou HA, Kuo YY, Liu CY, Chou WC, Lee MC, Chen CY, Lin LI, Tseng MH, Huang CF, Chiang YC, Lee FY, Liu MC, Liu CW, Tang JL, Yao M, Huang SY, Ko BS, Hsu SC, Wu SJ, Tsay W, Chen YC, Tien HF. Hou HA, et al. Blood. 2012 Jan 12;119(2):559-68. doi: 10.1182/blood-2011-07-369934. Epub 2011 Nov 10. Blood. 2012. PMID: 22077061 - Acute myeloid leukemia with DNMT3A mutations.
Li Y, Zhu B. Li Y, et al. Leuk Lymphoma. 2014 Sep;55(9):2002-12. doi: 10.3109/10428194.2013.869802. Epub 2014 Mar 7. Leuk Lymphoma. 2014. PMID: 24283755 Review. - DNMT3A R882 Mutations Predict a Poor Prognosis in AML: A Meta-Analysis From 4474 Patients.
Yuan XQ, Peng L, Zeng WJ, Jiang BY, Li GC, Chen XP. Yuan XQ, et al. Medicine (Baltimore). 2016 May;95(18):e3519. doi: 10.1097/MD.0000000000003519. Medicine (Baltimore). 2016. PMID: 27149454 Free PMC article. Review.
Cited by
- Mechanisms of epigenetic regulation of leukemia onset and progression.
Ntziachristos P, Mullenders J, Trimarchi T, Aifantis I. Ntziachristos P, et al. Adv Immunol. 2013;117:1-38. doi: 10.1016/B978-0-12-410524-9.00001-3. Adv Immunol. 2013. PMID: 23611284 Free PMC article. Review. - Molecular understanding of peripheral T-cell lymphomas, not otherwise specified (PTCL, NOS): A complex disease category.
Sakata-Yanagimoto M, Fukumoto K, Karube K, Chiba S. Sakata-Yanagimoto M, et al. J Clin Exp Hematop. 2021 Jun 5;61(2):61-70. doi: 10.3960/jslrt.20059. Epub 2021 Mar 15. J Clin Exp Hematop. 2021. PMID: 33716242 Free PMC article. Review. - Perturbed hematopoiesis in individuals with germline DNMT3A overgrowth Tatton-Brown-Rahman syndrome.
Tovy A, Rosas C, Gaikwad AS, Medrano G, Zhang L, Reyes JM, Huang YH, Arakawa T, Kurtz K, Conneely SE, Guzman AG, Aguilar R, Gao A, Chen CW, Kim JJ, Carter MT, Lasa-Aranzasti A, Valenzuela I, Van Maldergem L, Brunetti L, Hicks MJ, Marcogliese AN, Goodell MA, Rau RE. Tovy A, et al. Haematologica. 2022 Apr 1;107(4):887-898. doi: 10.3324/haematol.2021.278990. Haematologica. 2022. PMID: 34092059 Free PMC article. - Methylation-independent repression of Dnmt3b contributes to oncogenic activity of Dnmt3a in mouse MYC-induced T-cell lymphomagenesis.
Haney SL, Hlady RA, Opavska J, Klinkebiel D, Pirruccello SJ, Dutta S, Datta K, Simpson MA, Wu L, Opavsky R. Haney SL, et al. Oncogene. 2015 Oct;34(43):5436-5446. doi: 10.1038/onc.2014.472. Epub 2015 Feb 2. Oncogene. 2015. PMID: 25639876 Free PMC article. - Dnmt3b is a haploinsufficient tumor suppressor gene in Myc-induced lymphomagenesis.
Vasanthakumar A, Lepore JB, Zegarek MH, Kocherginsky M, Singh M, Davis EM, Link PA, Anastasi J, Le Beau MM, Karpf AR, Godley LA. Vasanthakumar A, et al. Blood. 2013 Mar 14;121(11):2059-63. doi: 10.1182/blood-2012-04-421065. Epub 2013 Jan 11. Blood. 2013. PMID: 23315164 Free PMC article.
References
- Paschka P, Schlenk RF, Gaidzik VI, et al. IDH1 and IDH2 mutations are frequent genetic alterations in acute myeloid leukemia and confer adverse prognosis in cytogenetically normal acute myeloid leukemia with NPM1 mutation without FLT3 internal tandem duplication. J Clin Oncol. 2010;28:3636–43. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- P01CA101937/CA/NCI NIH HHS/United States
- UL1 RR024992/RR/NCRR NIH HHS/United States
- P01 CA101937/CA/NCI NIH HHS/United States
- U54 HG003079/HG/NHGRI NIH HHS/United States
- P41 RR000954/RR/NCRR NIH HHS/United States
- P41RR000954/RR/NCRR NIH HHS/United States
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
Miscellaneous