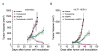Recombinant human erythropoietin antagonizes trastuzumab treatment of breast cancer cells via Jak2-mediated Src activation and PTEN inactivation - PubMed (original) (raw)
Comment
. 2010 Nov 16;18(5):423-35.
doi: 10.1016/j.ccr.2010.10.025.
Francisco J Esteva, Constance Albarracin, Katherine Stemke-Hale, Yang Lu, Giampaolo Bianchini, Ching-Yi Yang, Yong Li, Xinqun Li, Chun-Te Chen, Gordon B Mills, Gabriel N Hortobagyi, John Mendelsohn, Mien-Chie Hung, Zhen Fan
Affiliations
- PMID: 21075308
- PMCID: PMC3022383
- DOI: 10.1016/j.ccr.2010.10.025
Comment
Recombinant human erythropoietin antagonizes trastuzumab treatment of breast cancer cells via Jak2-mediated Src activation and PTEN inactivation
Ke Liang et al. Cancer Cell. 2010.
Abstract
We found that the receptor for erythropoietin (EpoR) is coexpressed with human epidermal growth factor receptor-2 (HER2) in a significant percentage of human breast tumor specimens and breast cancer cell lines. Exposure of HER2 and EpoR dual-positive breast cancer cells to recombinant human erythropoietin (rHuEPO) activated cell signaling. Concurrent treatment of the cells with rHuEPO and trastuzumab reduced the cells' response to trastuzumab both in vitro and in vivo. We identified Jak2-mediated activation of Src and inactivation of PTEN as underlying mechanisms through which rHuEPO antagonizes trastuzumab-induced therapeutic effects. Furthermore, we found that compared with administration of trastuzumab alone, concurrent administration of rHuEPO and trastuzumab correlated with shorter progression-free and overall survival in patients with HER2-positive metastatic breast cancer.
Copyright © 2010 Elsevier Inc. All rights reserved.
Figures
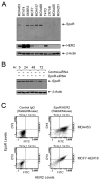
Figure 1. Coexpression of HER2 and EpoR in Human Breast Cancer Cell Lines
(A) Expression of HER2 and EpoR in human breast cancer cell lines. Exponentially proliferating breast cancer cells of the indicated lines were harvested by trypsinization. Equal amounts of cell lysates were subjected to Western blot analysis with specific antibodies directed against EpoR and HER2. The level of β-actin served as a protein-loading control. (B) Expression knockdown of EpoR by RNAi. MCF7 cells were subjected to EpoR expression knockdown with EpoR-specific Dharmacon SMARTpool siRNA or control siRNA for the indicated durations. Cell lysates were prepared, and the levels of EpoR were measured by Western blot analysis with EpoR-specific antibody. The level of β-actin served as a protein-loading control. (C) Coexpression of HER2 and EpoR. MDA453 and MCF7-HER18 cells were subjected to double-immunofluorescent staining with primary antibodies directed against EpoR (rabbit IgG) and HER2 (mouse IgG), followed by incubation with FITC-labeled goat anti-mouse IgG antibody and Cy3-labeled goat anti-rabbit IgG antibody. The cell suspensions were then analyzed by flow cytometry. See also Figure S1.
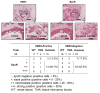
Figure 2. Expression of HER2 and EpoR in Human Breast Cancer Tissues from Patients
Two adjacent tissue slides from a whole-tissue paraffin block of a breast cancer specimen were stained immunohistochemically for the expressions of HER2 (left panel, stained brown with diaminobenzidine tetrahydrochloride) and EpoR (right panel, stained red with 3-aminoethyl-carbazole). The nuclei were counterstained with hematoxylin. Selected fields under low-power magnification (scale bar = 400 μm) were then viewed under high-power magnification (scale bar = 50 μm) and had the same pattern of HER2 and EpoR expression in ductal carcinoma in situ and in invasive cancer. The table summarizes findings from all 55 cases examined. See also Figure S2.
Figure 3. Activation of Cell Signaling by rHuEPO in EpoR-positive but Not in EpoR-undetectable Breast Cancer Cells
(A) Activation of cell signaling by rHuEPO in human breast cancer cell lines. The indicated cells were left untreated or were treated with 10 U/ml rHuEPO for 30 or 120 minutes in low-serum medium. Cell lysates were prepared, and equal amounts of cell lysates were subjected to Western blot analysis with antibodies directed against total and activation-specific phosphorylated Akt, Erk, and STAT5. The ratios represent quantitative analysis of densitometric values of specific band intensities normalized to the value of the corresponding untreated controls, which was arbitrarily set at 1. The level of β-actin served as a protein-loading control. (B) Dependence of rHuEPO-induced activation of cell signaling on EpoR expression. MCF7 cells were transfected for 72 hours with a control vector or one of two shRNA constructs targeting different regions of EpoR. The cells were stimulated with 10 U/ml rHuEPO for 30 minutes and immediately lysed for Western blot analysis with the indicated antibodies. (C) Effect of rHuEPO on cell signaling in additional breast cancer cell lines. The indicated cell lines were treated and analyzed as described in (A).
Figure 4. Antagonism by rHuEPO of Trastuzumab-Induced Inhibition of Cell Signaling and Antitumor Activities In Vitro
(A) Antagonism by rHuEPO of trastuzumab-induced inhibition of cell signaling. The indicated cell lines were left untreated or were treated with 20 nM trastuzumab, 10 U/ml rHuEPO, or both in low-serum medium overnight (16 hours). Cell lysates were prepared for Western blot analysis with the indicated antibodies. The ratios represent quantitative analysis of densitometric values of specific band intensities normalized to the value of the corresponding untreated controls, which was arbitrarily set at 1. (B) Antagonism by rHuEPO of trastuzumab-induced inhibition of cell growth. The indicated cell lines were treated as described in (A) for 5 days, and then an MTT colorimetric assay was performed to quantify relative survival and proliferation. The optical density value in each group of cells was directly plotted against the types of treatment. (C) Antagonism by rHuEPO of trastuzumab-induced inhibition of cell invasion and motility. The indicated cell lines were used in a Boyden transwell chamber assay (Becton Dickinson, Franklin Lakes, NJ) with the membrane coated with Matrigel. The cells were left untreated or treated with 20 nM trastuzumab, 10 U/ml rHuEPO, or both for 24 hours, and then the cells that had penetrated through the membrane were stained and counted using an inverted microscope equipped with a ×10 objective. The data are shown as the average number of cells per field. All scale bars represent 80 μm. (D) Antagonism by rHuEPO of trastuzumab-induced inhibition of cell clonogenic formation. MCF7-HER18 cells (500 cells per 60-mm dish) were exposed to trastuzumab in the presence or absence of 10 U/ml rHuEPO for 3 weeks. Numbers of cell colonies in trastuzumab-treated groups with or without current exposure to rHuEPO were normalized to the number of colonies in the control group (without any treatment).
Figure 5. Antagonism by rHuEPO of Trastuzumab-Induced Inhibition of Breast Cancer Xenograft Growth in Mouse Mammary Fat Pads
Starting on day 26 after inoculation of MDA453β cells into the mammary fat pads of female ICR SCID mice (4–6 weeks old, 5 mice/group) (A) and starting on day 20 after inoculation of MCF7-HER18/Fluc-GFP cells into the mammary fat pads of female Swiss nude mice (4–6 weeks old, 10 mice/group) (B), the mice were treated with PBS (control), trastuzumab (0.5 mg/mouse twice a week), epoetin alfa (100 U/mouse daily on weekdays), or trastuzumab plus epoetin alfa (same doses and schedules as each treatment alone) for 4 weeks or until mice were sacrificed, whichever came first. Tumor sizes were measured with a digital caliper every other day and plotted as a function of days after tumor cell inoculation. See also Figure S3.
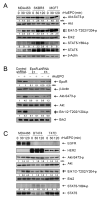
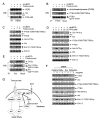
Figure 6. Roles of Jak2 and Src in Mediating rHuEPO-Induced Cell Signaling in HER2 and EpoR Dual-positive Breast Cancer Cells
(A and B) Dependence of rHuEPO-induced activation of cell signaling on Jak2 activity and expression. MCF7-HER18 cells were pre-exposed to 50 μM AG490 or DMSO in low-serum medium overnight (A) or subjected to expression knockdown of Jak2 by transient transfection with one of two different Jak2 shRNA constructs or control vector for 72 hours (B). The cells were then stimulated with 10 U/ml rHuEPO for 30 minutes, followed by cell lysis and Western blot analysis with the indicated antibodies. (C) Increased associations between Src and Jak2 and between Src and HER2 after rHuEPO stimulation. MCF7-HER18 and MDA453β cells were treated or untreated with 10 U/ml rHuEPO for 30 minutes or not. Cell lysates were subjected to immunoprecipitation with a Src antibody or mock antibody, followed by Western blot analysis with antibodies direct against Jak2 and HER2. (D and E) Role of Jak2 in rHuEPO-induced associations between Src and EpoR and between Src and HER2, and in rHuEPO-induced Src activation. MCF7-HER18 cells were transiently transfected with Jak2 shRNA constructs or control vector as described in (B) and then stimulated with 10 U/ml rHuEPO for 30 minutes. Src-immunoprecipitates (D) and whole cell lysates (E) were subjected to Western blot analysis with the indicated antibodies. See also Figure S4.
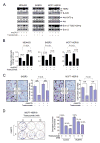
Figure 7. Roles of Src Activation and PTEN Inactivation in Conferring Resistance to Trastuzumab by rHuEPO
(A) Effects of rHuEPO and trastuzumab on association between Src and HER2. MCF7-HER18 cells were cultured in the presence or absence of 20 nM trastuzumab overnight in low serum medium and then stimulated with 10 U/ml rHuEPO or not for 30 minutes. The cell lysates were subjected to immunoprecipitation of HER2 with an anti-HER2 antibody, followed by Western blot analysis with the indicated antibodies. (B) Phosphorylation of PTEN upon rHuEPO stimulation. MCF7-HER18 cells were treated as described in (A). Cell lysates were prepared and subjected to immunoprecipitation for PTEN, followed by Western blot analysis with the indicated antibodies. (C) Correlation of rHuEPO-induced phosphorylation of PTEN with its antagonizing effects on trastuzumab-mediated inhibition of cell signaling. MCF7-HER18 cells were treated as described in (A). Cell lysates were subjected to Western blot analysis with the indicated antibodies. (D) Dependence of rHuEPO-induced cell signaling on Src activity. MCF7-HER18 cells were left untreated or treated with 5 μM or 25 μM PP2 (Src inhibitor) in 0.5% FBS medium overnight and then stimulated with 10 U/ml rHuEPO or not for 30 minutes, followed by cell lysis and Western blot analysis with the indicated antibodies. (E) Effects of rHuEPO and trastuzumab on Src and CK2α association. MCF7-HER18 cells were treated as described in (A). Cell lysates were prepared and subjected to immunoprecipitation of Src, followed by Western blot analysis of the immunoprecipitates with the indicated antibodies. (F) Roles of Src and CK2α in rHuEPO-mediated phosphorylation of Akt and Erk. MCF7-HER18 cells were transfected with siRNA for expression knockdown of CK2α, Src, or CK2α and Src, as indicated. After 72 hours, the cells were stimulated with 10 U/ml rHuEPO or not for 30 minutes, followed by cell lysis and Western blot analysis with the indicated antibodies. (G) Schematic of our working model. Trastuzumab binds to HER2 expressed by breast cancer cells, preventing activation of HER2-mediated cell signaling (dashed lines). rHuEPO binds to EpoR expressed by the same breast cancer cells, leading to activation of EpoR-associated protein tyrosine kinase Jak2 and subsequent activation of Src and inactivation of PTEN. The thick arrows indicate the pathways identified in the current study. The thick arrow with a question mark indicates a need for further confirmation. All of the experiments (A–F) were repeated at least once with similar findings. See also Figure S5.
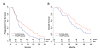
Figure 8. Progression-free Survival and Overall Survival of Patients with HER2-positive Metastatic Breast Cancer Treated with Trastuzumab-based Chemotherapy with or without Concurrent rHuEPO
(A) Progression-free survival. (B) Overall survival. See also Figure S6 and Tables S1 and S2.
Comment on
- Unfavorable drug interactions in targeted breast cancer therapy.
Groner B, Hynes NE. Groner B, et al. Cancer Cell. 2010 Nov 16;18(5):401-2. doi: 10.1016/j.ccr.2010.10.027. Cancer Cell. 2010. PMID: 21075302 No abstract available.
Similar articles
- Erythropoietin receptor expression and its relationship with trastuzumab response and resistance in HER2-positive breast cancer cells.
Zhang C, Duan X, Xu L, Ye J, Zhao J, Liu Y. Zhang C, et al. Breast Cancer Res Treat. 2012 Dec;136(3):739-48. doi: 10.1007/s10549-012-2316-x. Epub 2012 Nov 2. Breast Cancer Res Treat. 2012. PMID: 23117856 - Growth differentiation factor 15 (GDF15)-mediated HER2 phosphorylation reduces trastuzumab sensitivity of HER2-overexpressing breast cancer cells.
Joshi JP, Brown NE, Griner SE, Nahta R. Joshi JP, et al. Biochem Pharmacol. 2011 Nov 1;82(9):1090-9. doi: 10.1016/j.bcp.2011.07.082. Epub 2011 Jul 23. Biochem Pharmacol. 2011. PMID: 21803025 Free PMC article. - Loss of phosphatase and tensin homolog or phosphoinositol-3 kinase activation and response to trastuzumab or lapatinib in human epidermal growth factor receptor 2-overexpressing locally advanced breast cancers.
Dave B, Migliaccio I, Gutierrez MC, Wu MF, Chamness GC, Wong H, Narasanna A, Chakrabarty A, Hilsenbeck SG, Huang J, Rimawi M, Schiff R, Arteaga C, Osborne CK, Chang JC. Dave B, et al. J Clin Oncol. 2011 Jan 10;29(2):166-73. doi: 10.1200/JCO.2009.27.7814. Epub 2010 Dec 6. J Clin Oncol. 2011. PMID: 21135276 Free PMC article. - [Trastuzumab (Herceptin) and breast cancer: mechanisms of resistance].
Dieras V, Vincent-Salomon A, Degeorges A, Beuzeboc P, Mignot L, de Cremoux P. Dieras V, et al. Bull Cancer. 2007 Mar;94(3):259-66. Bull Cancer. 2007. PMID: 17371768 Review. French. - Mechanisms of disease: understanding resistance to HER2-targeted therapy in human breast cancer.
Nahta R, Yu D, Hung MC, Hortobagyi GN, Esteva FJ. Nahta R, et al. Nat Clin Pract Oncol. 2006 May;3(5):269-80. doi: 10.1038/ncponc0509. Nat Clin Pract Oncol. 2006. PMID: 16683005 Review.
Cited by
- Molecular pathways: molecular basis for sensitivity and resistance to JAK kinase inhibitors.
Meyer SC, Levine RL. Meyer SC, et al. Clin Cancer Res. 2014 Apr 15;20(8):2051-9. doi: 10.1158/1078-0432.CCR-13-0279. Epub 2014 Feb 28. Clin Cancer Res. 2014. PMID: 24583800 Free PMC article. Review. - Src family kinases, adaptor proteins and the actin cytoskeleton in epithelial-to-mesenchymal transition.
Ortiz MA, Mikhailova T, Li X, Porter BA, Bah A, Kotula L. Ortiz MA, et al. Cell Commun Signal. 2021 Jun 30;19(1):67. doi: 10.1186/s12964-021-00750-x. Cell Commun Signal. 2021. PMID: 34193161 Free PMC article. Review. - The two faces of Janus kinases and their respective STATs in mammary gland development and cancer.
Wagner KU, Schmidt JW. Wagner KU, et al. J Carcinog. 2011;10:32. doi: 10.4103/1477-3163.90677. Epub 2011 Dec 8. J Carcinog. 2011. PMID: 22279417 Free PMC article. - β2-AR signaling controls trastuzumab resistance-dependent pathway.
Liu D, Yang Z, Wang T, Yang Z, Chen H, Hu Y, Hu C, Guo L, Deng Q, Liu Y, Yu M, Shi M, Du N, Guo N. Liu D, et al. Oncogene. 2016 Jan 7;35(1):47-58. doi: 10.1038/onc.2015.58. Epub 2015 Mar 23. Oncogene. 2016. PMID: 25798840 - Widespread potential for growth-factor-driven resistance to anticancer kinase inhibitors.
Wilson TR, Fridlyand J, Yan Y, Penuel E, Burton L, Chan E, Peng J, Lin E, Wang Y, Sosman J, Ribas A, Li J, Moffat J, Sutherlin DP, Koeppen H, Merchant M, Neve R, Settleman J. Wilson TR, et al. Nature. 2012 Jul 26;487(7408):505-9. doi: 10.1038/nature11249. Nature. 2012. PMID: 22763448 Free PMC article.
References
- Belsches-Jablonski AP, Biscardi JS, Peavy DR, Tice DA, Romney DA, Parsons SJ. Src family kinases and HER2 interactions in human breast cancer cell growth and survival. Oncogene. 2001;20:1465–1475. - PubMed
- Berns K, Horlings HM, Hennessy BT, Madiredjo M, Hijmans EM, Beelen K, Linn SC, Gonzalez-Angulo AM, Stemke-Hale K, Hauptmann M, et al. A functional genetic approach identifies the PI3K pathway as a major determinant of trastuzumab resistance in breast cancer. Cancer Cell. 2007;12:395–402. - PubMed
- Bohlius J, Schmidlin K, Brillant C, Schwarzer G, Trelle S, Seidenfeld J, Zwahlen M, Clarke M, Weingart O, Kluge S, et al. Recombinant human erythropoiesis-stimulating agents and mortality in patients with cancer: a meta-analysis of randomised trials. Lancet. 2009;373:1532–1542. - PubMed
- Damen JE, Cutler RL, Jiao H, Yi T, Krystal G. Phosphorylation of tyrosine 503 in the erythropoietin receptor (EpR) is essential for binding the P85 subunit of phosphatidylinositol (PI) 3-kinase and for EpR-associated PI 3-kinase activity. J Biol Chem. 1995;270:23402–23408. - PubMed
- Damen JE, Liu L, Cutler RL, Krystal G. Erythropoietin stimulates the tyrosine phosphorylation of Shc and its association with Grb2 and a 145-Kd tyrosine phosphorylated protein. Blood. 1993a;82:2296–2303. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- P30 CA016672/CA/NCI NIH HHS/United States
- R01 CA129036-01A1/CA/NCI NIH HHS/United States
- R01 CA129036-02/CA/NCI NIH HHS/United States
- CA016672/CA/NCI NIH HHS/United States
- P50 CA098258/CA/NCI NIH HHS/United States
- R01 CA129036-03/CA/NCI NIH HHS/United States
- R01 CA129036/CA/NCI NIH HHS/United States
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
Research Materials
Miscellaneous