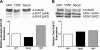Sprouty2 downregulates angiogenesis during mouse skin wound healing - PubMed (original) (raw)
Sprouty2 downregulates angiogenesis during mouse skin wound healing
Mateusz S Wietecha et al. Am J Physiol Heart Circ Physiol. 2011 Feb.
Abstract
Angiogenesis is regulated by signals received by receptor tyrosine kinases such as vascular endothelial growth factor receptors. Mammalian Sprouty (Spry) proteins are known to function by specifically antagonizing the activation of the mitogen-activated protein kinase signaling pathway by receptor tyrosine kinases, a pathway known to promote angiogenesis. To examine the role of Spry2 in the regulation of angiogenesis during wound repair, we used a model of murine dermal wound healing. Full-thickness excisional wounds (3 mm) were made on the dorsum of anesthetized adult female FVB mice. Samples were harvested at multiple time points postwounding and analyzed using real-time RT-PCR, Western blot analysis, and immunofluorescent histochemistry. Spry2 mRNA and protein levels in the wound bed increased significantly during the resolving phases of healing, coincident with the onset of vascular regression in this wound model. In another experiment, intracellular levels of Spry2 or its dominant-negative mutant (Y55F) were elevated by a topical application to the wounds of controlled-release gel containing cell permeable, transactivator of transcription-tagged Spry2, Spry2Y55F, or green fluorescent protein (as control). Wound samples were analyzed for vascularity using CD31 immunofluorescent histochemistry as well as for total and phospho-Erk1/2 protein content. The treatment of wounds with Spry2 resulted in a significant decrease in vascularity and a reduced abundance of phospho-Erk1/2 compared with wounds treated with the green fluorescent protein control. In contrast, the wounds treated with the dominant-negative Spry2Y55F exhibited a moderate increase in vascularity and elevated phospho-Erk1/2 content. These results indicate that endogenous Spry2 functions to downregulate angiogenesis in the healing murine skin wound, potentially by inhibiting the mitogen-activated protein kinase signaling pathway.
Figures
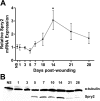
Fig. 1.
Sprouty 2 (Spry2) mRNA expression and protein levels in mouse skin wounds during healing. Wound samples were harvested at 8 time points following 3-mm dermal excisional punch biopsy and subjected to biochemical analysis. A: Spry2 mRNA transcript abundance was measured using real-time RT-PCR, normalized to GAPDH endogenous control at each time point, and compared with normal, unwounded skin (NS). Data are expressed as means ± SE; n = 5 for all time points except day 10 (n = 4). *P < 0.05 at day 14 vs. NS, when a peak in Spry2 mRNA is observed, by one-way ANOVA and Bonferroni's posttest. B: Western blot analysis shows that Spry2 protein levels follow the general pattern of Spry2 mRNA expression during wound healing. Separated bands were visualized using anti-human Spry2 and anti-α-tubulin antibodies. α-Tubulin was used as a loading control. Representative blot is from four independent experiments with similar results.
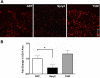
Fig. 2.
Spry2-positive cell numbers increase in the dermis of the wound bed during healing. Immunofluorescent histochemical analysis for Spry2 was performed on cryosections from whole wound samples following harvest at 8 time points after 3-mm dermal excisional punch biopsy. A: quantification of Spry2-positive cells in the wound bed (top) and wound margin (bottom). Data are expressed as means ± SE; n = 3 for all time points except days 1 and 5 (n = 2) and day 10 (n = 4). *P < 0.05 at day 28 vs. day 7 in the wound bed by one-way ANOVA and Bonferroni's posttest. B: representative photomicrographs of Spry2-positive immunofluorescence in the dermis of the wound bed from days 7, 14, and 28 postwounding; a negative control (NC) using a rabbit IgG primary antibody from a day 28 wound is shown. Scale bar = 50 μm.
Fig. 3.
Endothelial cell (EC) migration and mitogen-activated protein kinase (MAPK) signaling are inhibited following incubation with recombinant transactivator of transcription (TAT)-tagged Spry2. A: TAT-Spry2 inhibits the migration of mouse embryonic ECs in response to serum. Mouse embryonic ECs grown to confluency were pretreated with 20 μg/ml of TAT-proteins for 5 h before making scratch wounds and monitoring cell migration in response to serum (10%) while in the presence of TAT proteins, as described in
materials and methods
. Marked fields were photographed at time 0 and 15 h after making the scratches, and migration of cells was calculated as percent closure of scratch wound. The mean ± SE of 2 separate experiments is shown. *P < 0.01 for Spry2 vs. green fluorescent protein (GFP) in the serum group by one-way ANOVA and Bonferroni's posttest. B: TAT-Spry2 inhibits MAPK signaling of human umbilical vein ECs in response to vascular endothelial growth factor (VEGF). Human umbilical vein ECs were serum starved in endothelium growth medium-2 containing 0.1% FBS overnight and pretreated with 10 μg/ml each of TAT-GFP, TAT-Spry2, or TAT-Spry2Y55F for 1 h at 37°C before incubating with VEGF (50 ng/ml) for 10 or 30 min. Total cell lysates were subjected to SDS-PAGE and immunoblotted with anti-phospho-Erk1/2 (p-Erk1/2), anti-total Erk1/2 (t-Erk1/2), and anti-hemagglutinin (HA)-peroxidase (to detect TAT-tagged proteins)-conjugated antibodies.
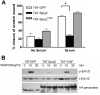
Fig. 4.
Wound vascularity is decreased following exogenous application of recombinant TAT-tagged Spry2. Immunofluorescent histochemical analysis for EC marker CD31 (platelet EC adhesion molecule-1) was performed on cryosections from whole wound samples harvested at day 10 postinjury from 3-mm dermal excisional punch biopsy and after exogenous application at day 5 postwounding of controlled-release gel containing 2 μg of recombinant cell-permeable, TAT-tagged GFP (control), Spry2, or dominant-negative mutant of Spry2 (Y55F) (n = 6 for GFP, n = 5 for Spry2, and n = 6 for Spry2Y55F). A: representative photomicrographs of CD31-positive immunofluorescence in GFP-, Spry2-, and Spry2Y55F-treated wounds. Scale bar = 50 μm. B: quantification of CD31 immunofluorescence shows a moderate increase in vascularity in Spry2Y55F-treated wounds and a significant decrease in vascularity in Spry2-treated wounds relative to the GFP-treated control group. Percent CD31 area per field was normalized and compared with the GFP control group to yield fold change in CD31 area; data are expressed as means ± SE_. *P_ = 0.05 for Spry2 vs. GFP by one-way ANOVA and Bonferroni's posttest.
Fig. 5.
MAPK signaling is decreased in TAT-Spry2-treated wounds, whereas TAT-Spry2Y55F-treated wounds exhibit increased MAPK signaling. Levels of signaling proteins were analyzed by Western blot analysis performed on whole wound samples harvested at day 10 postinjury from 3-mm dermal excisional punch biopsy and after exogenous treatment with TAT-tagged recombinant proteins at day 5 postwounding. Data represent results from 1 of 4 independent experiments with similar results. A and B, top: representative Western blots showing expression of p-Erk1/2 (A) and t-Erk1/2 (B) signaling proteins in GFP-, Spry2Y55F-, Spry2-treated wounds. α-Tubulin was used as a loading control. A and B, bottom: quantification of respective Western blots showing p-Erk1/2 (A) and t-Erk1/2 (B) protein expression normalized to α-tubulin and compared with the GFP control group.
Fig. 6.
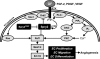
Spry2 and Spry2Y55F function in the context of EC MAPK signaling. Upon growth factor (GF) binding to its respective receptor tyrosine kinase (RTK) and activation of the respective signaling pathway, Spry2 is induced to translocate to the inner plasma membrane where it gets activated (in many cases via phosphorylation on the Y55 by a Src-like kinase) and functions by interacting with various MAPK signaling pathway-associated proteins in a GF-specific manner. When RTK signaling is Ras dependent (left), Spry2 inhibition of this pathway is thought to occur at the level of GF receptor-bound protein-2 (Grb2) or Raf1. When RTK signaling is Ras independent (right), Spry2 inhibition of this pathway is thought to occur at the level of Raf1. In many cases, pY55 is required for Spry2 inhibition of the Raf/Mek/Erk pathway. The dominant-negative Y55F mutant of Spry2 inhibits endogenous Spry2 action, thereby promoting the Raf/Mek/Erk pathway. FGF-2, fibroblast GF-2; PDGF, platelet-derived GF; pY, phosphorylated tyrosine; Sos1, son of sevenless-1; PLC-γ, phospholipase C-γ; PIP2, phosphatidylinositol bisphosphate; DAG, diacylglycerol; IP3, inositol trisphosphate; PKC, protein kinase C. Information in this figure was compiled from Refs. , , , , , , , , .
Similar articles
- Increased angiogenesis and expression of vascular endothelial growth factor during scarless repair.
Colwell AS, Beanes SR, Soo C, Dang C, Ting K, Longaker MT, Atkinson JB, Lorenz HP. Colwell AS, et al. Plast Reconstr Surg. 2005 Jan;115(1):204-12. Plast Reconstr Surg. 2005. PMID: 15622252 - The Role of the miR-21/SPRY2 Axis in Modulating Proangiogenic Factors, Epithelial Phenotypes, and Wound Healing in Corneal Epithelial Cells.
Zhang Y, Yuan F, Liu L, Chen Z, Ma X, Lin Z, Zou J. Zhang Y, et al. Invest Ophthalmol Vis Sci. 2019 Sep 3;60(12):3854-3862. doi: 10.1167/iovs.19-27013. Invest Ophthalmol Vis Sci. 2019. PMID: 31529118 Free PMC article. - Elevation of hemopexin-like fragment of matrix metalloproteinase-2 tissue levels inhibits ischemic wound healing and angiogenesis.
Nedeau AE, Gallagher KA, Liu ZJ, Velazquez OC. Nedeau AE, et al. J Vasc Surg. 2011 Nov;54(5):1430-8. doi: 10.1016/j.jvs.2011.05.029. Epub 2011 Sep 8. J Vasc Surg. 2011. PMID: 21903356 Free PMC article. - SPROUTY2, a Negative Feedback Regulator of Receptor Tyrosine Kinase Signaling, Associated with Neurodevelopmental Disorders: Current Knowledge and Future Perspectives.
Puranik N, Jung H, Song M. Puranik N, et al. Int J Mol Sci. 2024 Oct 14;25(20):11043. doi: 10.3390/ijms252011043. Int J Mol Sci. 2024. PMID: 39456824 Free PMC article. Review. - Novel regulatory mechanisms underlying angiogenesis during wound healing revealed by fluorescence-based live-imaging in zebrafish.
Yuge S, Ishii T, Noishiki C, Fukuhara S. Yuge S, et al. J Biochem. 2023 Jun 30;174(1):5-12. doi: 10.1093/jb/mvad024. J Biochem. 2023. PMID: 36931281 Review.
Cited by
- Cellular and molecular mechanisms of skin wound healing.
Peña OA, Martin P. Peña OA, et al. Nat Rev Mol Cell Biol. 2024 Aug;25(8):599-616. doi: 10.1038/s41580-024-00715-1. Epub 2024 Mar 25. Nat Rev Mol Cell Biol. 2024. PMID: 38528155 Review. - Inhibition of Sprouty2 polarizes macrophages toward an M2 phenotype by stimulation with interferon γ and Porphyromonas gingivalis lipopolysaccharide.
Atomura R, Sanui T, Fukuda T, Tanaka U, Toyoda K, Taketomi T, Yamamichi K, Akiyama H, Nishimura F. Atomura R, et al. Immun Inflamm Dis. 2016 Feb 26;4(1):98-110. doi: 10.1002/iid3.99. eCollection 2016 Mar. Immun Inflamm Dis. 2016. PMID: 27042307 Free PMC article. - Nod-like receptor protein-3 inflammasome plays an important role during early stages of wound healing.
Weinheimer-Haus EM, Mirza RE, Koh TJ. Weinheimer-Haus EM, et al. PLoS One. 2015 Mar 20;10(3):e0119106. doi: 10.1371/journal.pone.0119106. eCollection 2015. PLoS One. 2015. PMID: 25793779 Free PMC article. - Angiogenesis in Wound Repair: Too Much of a Good Thing?
Han C, Barakat M, DiPietro LA. Han C, et al. Cold Spring Harb Perspect Biol. 2022 Oct 3;14(10):a041225. doi: 10.1101/cshperspect.a041225. Cold Spring Harb Perspect Biol. 2022. PMID: 35667793 Free PMC article. Review. - Tumour-Derived Laminin α5 (LAMA5) Promotes Colorectal Liver Metastasis Growth, Branching Angiogenesis and Notch Pathway Inhibition.
Gordon-Weeks A, Lim SY, Yuzhalin A, Lucotti S, Vermeer JAF, Jones K, Chen J, Muschel RJ. Gordon-Weeks A, et al. Cancers (Basel). 2019 May 6;11(5):630. doi: 10.3390/cancers11050630. Cancers (Basel). 2019. PMID: 31064120 Free PMC article.
References
- Alon T, Hemo I, Itin A, Pe'er J, Stone J, Keshet E. Vascular endothelial growth factor acts as a survival factor for newly formed retinal vessels and has implications for retinopathy of prematurity. Nat Med 1: 1024–1028, 1995 - PubMed
- Antoine M, Wirz W, Tag CG, Mavituna M, Emans N, Korff T, Stoldt V, Gressner AM, Kiefer P. Expression pattern of fibroblast growth factors (FGFs), their receptors and antagonists in primary endothelial cells and vascular smooth muscle cells. Growth Factors 23: 87–95, 2005 - PubMed
- Ballaun C, Weninger W, Uthman A, Weich H, Tschachler E. Human keratinocytes express the three major splice forms of vascular endothelial growth factor. J Invest Dermatol 104: 7–10, 1995 - PubMed
- Barrientos S, Stojadinovic O, Golinko MS, Brem H, Tomic-Canic M. Growth factors and cytokines in wound healing. Wound Repair Regen 16: 585–601, 2008 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- T32-AA-013527/AA/NIAAA NIH HHS/United States
- R01 GM050875/GM/NIGMS NIH HHS/United States
- F30 DE020991/DE/NIDCR NIH HHS/United States
- R01-GM-50875/GM/NIGMS NIH HHS/United States
- R01 GM073181/GM/NIGMS NIH HHS/United States
- P20-GM-078426/GM/NIGMS NIH HHS/United States
- T32-DE-018381/DE/NIDCR NIH HHS/United States
- R01-GM-073181/GM/NIGMS NIH HHS/United States
LinkOut - more resources
Full Text Sources
Miscellaneous