Vascular smooth muscle cell peroxisome proliferator-activated receptor-γ mediates pioglitazone-reduced vascular lesion formation - PubMed (original) (raw)
Vascular smooth muscle cell peroxisome proliferator-activated receptor-γ mediates pioglitazone-reduced vascular lesion formation
Milton Hamblin et al. Arterioscler Thromb Vasc Biol. 2011 Feb.
Abstract
Objective: Peroxisome proliferator-activated receptor-γ (PPARγ) has been reported to decrease vascular lesion formation. However, the critical role of vascular smooth muscle cell (VSMC) PPARγ in vascular lesion formation following transplantation is not well understood. In this study, we investigated the role of VSMC PPARγ-mediated signaling in transplantation-associated vascular lesion formation.
Methods and results: Carotid arteries from smooth muscle cell-selective PPARγ knockout (SMPG KO) and wild-type mice were transplanted to CBA/CaJ recipient mice. The recipient mice received a control diet or pioglitazone-containing diet. Pioglitazone reduced vascular lesion formation in transplanted wild-type, but not in SMPG KO carotid arteries. Histological analysis suggested that PPARγ attenuates vascular lesion formation through antiinflammatory signaling, as evidenced by the increase of intimal inflammatory cells and tumor necrosis factor-α expression in SMPG KO allografts. Intravital microscopy revealed increased inflammatory cell rolling and attachment to endothelial cells in small blood vessels of SMPG KO mice following cytokine stimulation. SMPG KO mice, as shown by Western blotting, have elevated vascular cell adhesion molecule-1 (VCAM-1) expression. Furthermore, immunohistochemistry demonstrated SMPG KO allografts have increased VCAM-1.
Conclusions: Loss of PPARγ in VSMC promotes transplantation-associated vascular lesion formation through increased VCAM-1 expression. VSMC PPARγ also mediates pioglitazone-reduced vascular lesion formation.
Figures
Figure 1. Effects of VSMC PPARγ on vascular lesion formation
(A) Representative histological sections of carotid arteries from VSMC-selective PPARγ knockout (SMPG KO) and wild-type mice that were transplanted to CBA/CaJ recipient mice administered either a pioglitazone-treated diet or normal diet. Scale bars= 50 μm. Thin arrows demonstrate internal elastic layer and thick arrows demonstrate external elastic layer. (B) The effect of pioglitazone on the neointimal area of SMPG KO and wild-type mouse allografts. (C) The effect of pioglitazone on the neointimal area/medial area of SMPG KO and wild-type mouse allografts. n=6 for SMPG KO group and n=7 for wild-type group. Results are mean ± SEM. _P_-values < 0.05 were considered statistically significant.
Figure 2. VSMC PPARγ deletion increases inflammatory cell attachment
Representative hematoxylin and eosin staining of VSMC-selective PPARγ knockout (SMPG KO) and wild-type mouse carotid arteries that were transplanted to CBA/CaJ recipient mice fed a normal diet. Two weeks after transplantation, there was greater inflammatory cell accumulation in the lumens of SMPG KO allografts (B) compared with wild-type allografts (A). n=6 for SMPG KO group and n=7 for wild-type group. The inflammatory cell number for SMPG KO tissue was greater compared with the inflammatory cell number for wild-type tissue (C). Immunohistochemical staining for TNF-α expression revealed a greater area positive for cytokine expression in SMPG KO (E, F) compared with wild-type vessels (D, F). n=3 for each group in immunohistochemical study. Two slides per animal were analyzed for TNF-α expression. Scale bars= 50 μm. Results are mean ± SEM. _P_-values < 0.05 were considered statistically significant.
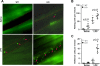
Figure 3. VSMC PPARγ deletion increases monocyte adhesion in vivo
Intravital microscopy was used for in vivo detection of monocytes labeled with rhodamine 6G. (A) Visualization of monocytes adhering to the venular endothelium of vascular smooth muscle cell PPARγ knockout (SMPG KO) and wild-type mice four hours after a 1 mg/kg lipopolysaccharide (LPS) injection. (B) Mice deficient in VSMC PPARγ displayed increases in the number of monocytes rolling on venular endothelium per minute in both saline- and LPS-treated studies. (C) LPS-injected SMPG KO mice demonstrated a significantly greater number of cells adhering to venular endothelium per minute versus wild-type mice. Data are representative of 6 separate experiments. Arrows represent rhodamine 6G-stained monocytes.
Figure 4. PPARγ affects VCAM-1 levels in VSMC
(A) Western Blot analysis revealed elevated VCAM-1 expression in thoracic arteries of eight-week-old smooth muscle cell-selective PPARγ knockout (SMPG KO) mice. n=3 for each pooled sample. (B, C) SMPG KO allografts, as shown by immunohistochemical staining, demonstrated a greater area of positive for VCAM-1 at two and four weeks after transplantation. n=3 animals for each group in immunohistochemical study. Two slides per animal were analyzed for VCAM-1 expression. (D) PPARγ overexpression by adenovirus (Ad-PPARγ) resulted in decreased VCAM-1 expression in rat aortic smooth muscle cells (RASMC) stimulated with 10 ng/ml hIL-1. In contrast, adenoviral PPARγ knockdown elevated VCAM-1 expression in hIL-1-stimulated cells. Ad-GFP was utilized as a control. (E) The addition of pioglitazone decreased hIL-1-induced increases in RASMC VCAM-1 expression. Results are mean ± SEM. _P_-values < 0.05 were considered statistically significant. Data are representative of 3 separate experiments.
Figure 5. PPARγ knockdown increases NF-κB activation in VSMC
(A) Western Blot analysis of cytosolic and nuclear p65 levels in hIL-stimulated (10 ng/ml) rat aortic smooth muscle cells (RASMC). PPARγ overexpression reduced while PPARγ knockdown increased nuclear p65 levels. Neither PPARγ overexpression nor PPARγ RNAi had any significant effect on cytosolic p65 levels. (B) Western Blot analysis of RASMC nuclear fractions showed PPARγ knockdown increased phosphorylation of both IkappaB kinase (IKK) and IkappaBalpha (IκBα). Ad-GFP served as an adenovirus control. Results are mean ± SEM. _P_-values < 0.05 were considered statistically significant. Data are representative of 3 separate experiments.
Similar articles
- Vascular smooth muscle cell peroxisome proliferator-activated receptor-γ deletion promotes abdominal aortic aneurysms.
Hamblin M, Chang L, Zhang H, Yang K, Zhang J, Chen YE. Hamblin M, et al. J Vasc Surg. 2010 Oct;52(4):984-93. doi: 10.1016/j.jvs.2010.05.089. Epub 2010 Jul 13. J Vasc Surg. 2010. PMID: 20630681 Free PMC article. - Smooth Muscle Peroxisome Proliferator-Activated Receptor γ Plays a Critical Role in Formation and Rupture of Cerebral Aneurysms in Mice In Vivo.
Hasan DM, Starke RM, Gu H, Wilson K, Chu Y, Chalouhi N, Heistad DD, Faraci FM, Sigmund CD. Hasan DM, et al. Hypertension. 2015 Jul;66(1):211-20. doi: 10.1161/HYPERTENSIONAHA.115.05332. Epub 2015 Apr 27. Hypertension. 2015. PMID: 25916724 Free PMC article. - PPARγ attenuates intimal hyperplasia by inhibiting TLR4-mediated inflammation in vascular smooth muscle cells.
Zhang LL, Gao CY, Fang CQ, Wang YJ, Gao D, Yao GE, Xiang J, Wang JZ, Li JC. Zhang LL, et al. Cardiovasc Res. 2011 Dec 1;92(3):484-93. doi: 10.1093/cvr/cvr238. Epub 2011 Aug 31. Cardiovasc Res. 2011. PMID: 21880694 - Anti-inflammatory mechanisms of the vascular smooth muscle PPARγ.
Mukohda M, Ozaki H. Mukohda M, et al. J Smooth Muscle Res. 2021;57(0):1-7. doi: 10.1540/jsmr.57.1. J Smooth Muscle Res. 2021. PMID: 33658456 Free PMC article. Review. - Activation of the Metabolic Master Regulator PPARγ: A Potential PIOneering Therapy for Pulmonary Arterial Hypertension.
Hansmann G, Calvier L, Risbano MG, Chan SY. Hansmann G, et al. Am J Respir Cell Mol Biol. 2020 Feb;62(2):143-156. doi: 10.1165/rcmb.2019-0226PS. Am J Respir Cell Mol Biol. 2020. PMID: 31577451 Free PMC article. Review.
Cited by
- Disruption of endothelial peroxisome proliferator-activated receptor γ accelerates diet-induced atherogenesis in LDL receptor-null mice.
Qu A, Shah YM, Manna SK, Gonzalez FJ. Qu A, et al. Arterioscler Thromb Vasc Biol. 2012 Jan;32(1):65-73. doi: 10.1161/ATVBAHA.111.239137. Epub 2011 Oct 20. Arterioscler Thromb Vasc Biol. 2012. PMID: 22015658 Free PMC article. - Endothelial dysfunction and cardiac allograft vasculopathy.
Colvin-Adams M, Harcourt N, Duprez D. Colvin-Adams M, et al. J Cardiovasc Transl Res. 2013 Apr;6(2):263-77. doi: 10.1007/s12265-012-9414-3. Epub 2012 Nov 8. J Cardiovasc Transl Res. 2013. PMID: 23135991 Review. - Molecular targets related to inflammation and insulin resistance and potential interventions.
Hirabara SM, Gorjão R, Vinolo MA, Rodrigues AC, Nachbar RT, Curi R. Hirabara SM, et al. J Biomed Biotechnol. 2012;2012:379024. doi: 10.1155/2012/379024. Epub 2012 Sep 25. J Biomed Biotechnol. 2012. PMID: 23049242 Free PMC article. Review. - Autologous fat transplants to deliver glitazone and adiponectin for vasculoprotection.
Sanders WG, Li H, Zhuplatov I, He Y, Kim SE, Cheung AK, Agarwal J, Terry CM. Sanders WG, et al. J Control Release. 2017 Oct 28;264:237-246. doi: 10.1016/j.jconrel.2017.08.036. Epub 2017 Sep 1. J Control Release. 2017. PMID: 28867378 Free PMC article. - Pioglitazone Ameliorates Smooth Muscle Cell Proliferation in Cuff-Induced Neointimal Formation by Both Adiponectin-Dependent and -Independent Pathways.
Kubota T, Kubota N, Sato H, Inoue M, Kumagai H, Iwamura T, Takamoto I, Kobayashi T, Moroi M, Terauchi Y, Tobe K, Ueki K, Kadowaki T. Kubota T, et al. Sci Rep. 2016 Oct 5;6:34707. doi: 10.1038/srep34707. Sci Rep. 2016. PMID: 27703271 Free PMC article.
References
- Hamblin M, Chang L, Zhang J, Chen YE. The role of peroxisome proliferator-activated receptor gamma in blood pressure regulation. Curr Hypertens Rep. 2009;11:239–245. - PubMed
- Duan SZ, Ivashchenko CY, Russell MW, Milstone DS, Mortensen RM. Cardiomyocyte-specific knockout and agonist of peroxisome proliferator-activated receptor-gamma both induce cardiac hypertrophy in mice. Circ Res. 2005;97:372–379. - PubMed
- Ding G, Fu M, Qin Q, Lewis W, Kim HW, Fukai T, Bacanamwo M, Chen YE, Schneider MD, Mangelsdorf DJ, Evans RM, Yang Q. Cardiac peroxisome proliferator-activated receptor gamma is essential in protecting cardiomyocytes from oxidative damage. Cardiovasc Res. 2007;76:269–279. - PubMed
- Chawla A, Boisvert WA, Lee CH, Laffitte BA, Barak Y, Joseph SB, Liao D, Nagy L, Edwards PA, Curtiss LK, Evans RM, Tontonoz P. A PPAR gamma-LXR-ABCA1 pathway in macrophages is involved in cholesterol efflux and atherogenesis. Mol Cell. 2001;7:161–171. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- R01 HL068878-08S1/HL/NHLBI NIH HHS/United States
- T32 HL007853/HL/NHLBI NIH HHS/United States
- R01 HL068878/HL/NHLBI NIH HHS/United States
- R01 HL068878-08/HL/NHLBI NIH HHS/United States
- HL89544/HL/NHLBI NIH HHS/United States
- HL68878/HL/NHLBI NIH HHS/United States
- R21 HL092421/HL/NHLBI NIH HHS/United States
- R01 HL068878-09/HL/NHLBI NIH HHS/United States
- R01 HL105114/HL/NHLBI NIH HHS/United States
- R01 HL068878-10/HL/NHLBI NIH HHS/United States
- R21 HL092421-02/HL/NHLBI NIH HHS/United States
- R01 HL089544-03/HL/NHLBI NIH HHS/United States
- R01 HL089544-02/HL/NHLBI NIH HHS/United States
- R01 HL089544-02S1/HL/NHLBI NIH HHS/United States
- R01 HL089544/HL/NHLBI NIH HHS/United States
- R01 HL075397-05/HL/NHLBI NIH HHS/United States
- R01 HL105114-01/HL/NHLBI NIH HHS/United States
- R01 HL068878-09W1/HL/NHLBI NIH HHS/United States
- R01 HL075397/HL/NHLBI NIH HHS/United States
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Research Materials
Miscellaneous