The interleukin 13 (IL-13) pathway in human macrophages is modulated by microRNA-155 via direct targeting of interleukin 13 receptor alpha1 (IL13Ralpha1) - PubMed (original) (raw)
The interleukin 13 (IL-13) pathway in human macrophages is modulated by microRNA-155 via direct targeting of interleukin 13 receptor alpha1 (IL13Ralpha1)
Rocio T Martinez-Nunez et al. J Biol Chem. 2011.
Abstract
Macrophages play a central role in the balance and efficiency of the immune response and are at the interface between innate and adaptive immunity. Their phenotype is a delicate equilibrium between the M1 (classical, pro-Th(1)) and M2 (alternative, pro-Th(2)) profiles. This balance is regulated by cytokines such as interleukin 13 (IL-13), a typical pro-M2-Th(2) cytokine that has been related to allergic disease and asthma. IL-13 binds to IL-13 receptor α1 (IL13Rα1), a component of the Type II IL-4 receptor, and exerts its effects by activating the transcription factor signal transducer and activator of transcription 6 (STAT6) through phosphorylation. MicroRNAs are short (∼22 nucleotide) inhibitory non-coding RNAs that block the translation or promote the degradation of their specific mRNA targets. By bioinformatics analysis, we found that microRNA-155 (miR-155) is predicted to target IL13Rα1. This suggested that miR-155 might be involved in the regulation of the M1/M2 balance in macrophages by modulating IL-13 effects. miR-155 has been implicated in the development of a healthy immune system and function as well as in the inflammatory pro-Th(1)/M1 immune profile. Here we have shown that in human macrophages, miR-155 directly targets IL13Rα1 and reduces the levels of IL13Rα1 protein, leading to diminished activation of STAT6. Finally we also demonstrate that miR-155 affects the IL-13-dependent regulation of several genes (SOCS1, DC-SIGN, CCL18, CD23, and SERPINE) involved in the establishment of an M2/pro-Th(2) phenotype in macrophages. Our work shows a central role for miR-155 in determining the M2 phenotype in human macrophages.
Figures
FIGURE 1.
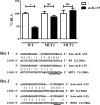
miR-155 directly targets the 3′-UTR of IL13Rα1. HeLa cells were co-transfected with a Renilla luciferase construct harboring an IL13Rα1 3′-UTR fragment containing the predicted binding sites for miR-155 (wild type, WT) and either an empty expression vector (−) or an miR-155-overexpressing vector (miR-155). MUT1 and MUT2 correspond to mutants in each one of the predicted sites, site 1 and site 2, respectively. One of three independent experiments is shown. ns = not significant, *, p ≤ 0.05. Error bars indicate S.D. RLA, relative luciferase activity.
FIGURE 2.
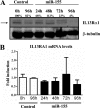
Overexpression of miR-155 reduces the levels of IL13Rα1 protein. THP1-155 cells were treated with doxycycline (miR-155) or not (Control) during the course of 96 h to allow miR-155 overexpression. Cells were collected in intervals of 24 h and subjected to protein and RNA extraction. A, cell lysates were subjected to Western blotting for IL13Rα1 protein detection (upper panel, lower band indicated by arrow) and normalized against β-tubulin (lower panel). B, total RNA was extracted, and mRNA levels of IL13Rα1 were determined by RT-qPCR. Shown is one experiment out of three independent ones. Statistical analysis of Western blots is shown in
supplemental Fig. S5
. Error bars indicate S.D.
FIGURE 3.
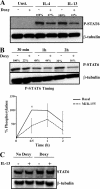
Overexpression of miR-155 reduces STAT6 phosphorylation. THP1-155 cells were treated with doxycycline (+Doxy) or not (−Doxy) during 96 h to overexpress miR-155. Cells were then starved overnight and stimulated with either IL-4 or IL-13 or not stimulated (Control) and lysed at the indicated times. A, analysis of STAT6 phosphorylation (P-STAT6) after 30 min of treatment was performed by Western blotting and normalized against β-tubulin. B, THP1-155 cells were stimulated with IL-13 or not, collected after 30 min, 1 h, and 2 h, and subjected to Western blotting. The lower panel shows the percentage of STAT6 phosphorylation (P-STAT6) in this panel plotted against time of treatment as analyzed by densitometry (three independent experiments shown, *, p ≤ 0.05). Error bars indicate S.D. C, THP1-155 cells treated or not with doxycycline for 96 h (overexpressing or not miR-155, respectively) were subjected to analysis of total STAT6 content by Western blotting and normalized against β-tubulin expression. Shown is one experiment out of three independent ones. Statistical analysis of Western blots is shown in
supplemental Fig. S6
.
FIGURE 4.
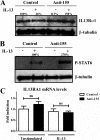
miR-155 down-regulation increases IL13Rα1 protein expression and STAT6 phosphorylation. In a reverse model in human macrophages, cells were transfected with blocking oligonucleotides against miR-155 (Anti-155) or a negative control (Control). On day 3 of culture macrophages were stimulated with IL-13 or not and collected after 30 min. A, cell lysates were subjected to Western blotting to detect IL13Rα1 normalized against β-tubulin. B, cell lysates were used to determine STAT6 phosphorylation (P-STAT6) levels normalizing against β-tubulin. C, RNA was extracted from the same collected cells, and mRNA of IL13Rα1 was determined by RT-qPCR. One of three independent experiments is shown. ns = not significant. Shown is one experiment out of three independent ones. Statistical analysis of Western blots is shown in
supplemental Figs. S7 and S8
. Error bars indicate S.D.
FIGURE 5.
Down-regulation of miR-155 increases the transcription of several STAT6/IL-13-dependent genes. Human macrophages were transfected with anti-miR-155 oligonucleotides (Anti-155) or a negative control (Control). On day 3 of culture, cells were stimulated with or without IL-13 and collected 24 h after stimulation to analyze mRNA expression by RT-qPCR analysis. The genes assayed were grouped as genes dependent on the IL-13/STAT6 signaling, CCL18, SOCS1, CD23, SERPINE, and DC-SIGN (A) and as genes not affected by IL-13 treatment, TGFB1 and IL-10 (B). One of three independent experiments is shown. ns = not significant, *, p ≤ 0.05, **, p ≤ 0.01. Error bars indicate S.D.
Similar articles
- miR-143 inhibits interleukin-13-induced inflammatory cytokine and mucus production in nasal epithelial cells from allergic rhinitis patients by targeting IL13Rα1.
Teng Y, Zhang R, Liu C, Zhou L, Wang H, Zhuang W, Huang Y, Hong Z. Teng Y, et al. Biochem Biophys Res Commun. 2015 Jan 30;457(1):58-64. doi: 10.1016/j.bbrc.2014.12.058. Epub 2014 Dec 18. Biochem Biophys Res Commun. 2015. PMID: 25529447 - LncGBP9/miR-34a axis drives macrophages toward a phenotype conducive for spinal cord injury repair via STAT1/STAT6 and SOCS3.
Zhou J, Li Z, Wu T, Zhao Q, Zhao Q, Cao Y. Zhou J, et al. J Neuroinflammation. 2020 Apr 28;17(1):134. doi: 10.1186/s12974-020-01805-5. J Neuroinflammation. 2020. PMID: 32345320 Free PMC article. - The Interleukin-13 Receptor-α1 Chain Is Essential for Induction of the Alternative Macrophage Activation Pathway by IL-13 but Not IL-4.
Sheikh F, Dickensheets H, Pedras-Vasconcelos J, Ramalingam T, Helming L, Gordon S, Donnelly RP. Sheikh F, et al. J Innate Immun. 2015;7(5):494-505. doi: 10.1159/000376579. Epub 2015 Mar 7. J Innate Immun. 2015. PMID: 25766112 Free PMC article. - MiRNA-Mediated Macrophage Polarization and its Potential Role in the Regulation of Inflammatory Response.
Essandoh K, Li Y, Huo J, Fan GC. Essandoh K, et al. Shock. 2016 Aug;46(2):122-31. doi: 10.1097/SHK.0000000000000604. Shock. 2016. PMID: 26954942 Free PMC article. Review. - Investigational therapeutics targeting the IL-4/IL-13/STAT-6 pathway for the treatment of asthma.
Oh CK, Geba GP, Molfino N. Oh CK, et al. Eur Respir Rev. 2010 Mar;19(115):46-54. doi: 10.1183/09059180.00007609. Eur Respir Rev. 2010. PMID: 20956165 Free PMC article. Review.
Cited by
- Physiological roles of miR-155.
Mashima R. Mashima R. Immunology. 2015 Jul;145(3):323-33. doi: 10.1111/imm.12468. Epub 2015 May 19. Immunology. 2015. PMID: 25829072 Free PMC article. Review. - MicroRNAs are universal regulators of differentiation, activation, and polarization of microglia and macrophages in normal and diseased CNS.
Ponomarev ED, Veremeyko T, Weiner HL. Ponomarev ED, et al. Glia. 2013 Jan;61(1):91-103. doi: 10.1002/glia.22363. Epub 2012 May 31. Glia. 2013. PMID: 22653784 Free PMC article. Review. - miR-aculous new avenues for cancer immunotherapy.
Tang WW, Bauer KM, Barba C, Ekiz HA, O'Connell RM. Tang WW, et al. Front Immunol. 2022 Sep 28;13:929677. doi: 10.3389/fimmu.2022.929677. eCollection 2022. Front Immunol. 2022. PMID: 36248881 Free PMC article. Review. - MicroRNA-155 is required for effector CD8+ T cell responses to virus infection and cancer.
Dudda JC, Salaun B, Ji Y, Palmer DC, Monnot GC, Merck E, Boudousquie C, Utzschneider DT, Escobar TM, Perret R, Muljo SA, Hebeisen M, Rufer N, Zehn D, Donda A, Restifo NP, Held W, Gattinoni L, Romero P. Dudda JC, et al. Immunity. 2013 Apr 18;38(4):742-53. doi: 10.1016/j.immuni.2012.12.006. Immunity. 2013. PMID: 23601686 Free PMC article. - miR-155 Deletion in Mice Overcomes Neuron-Intrinsic and Neuron-Extrinsic Barriers to Spinal Cord Repair.
Gaudet AD, Mandrekar-Colucci S, Hall JC, Sweet DR, Schmitt PJ, Xu X, Guan Z, Mo X, Guerau-de-Arellano M, Popovich PG. Gaudet AD, et al. J Neurosci. 2016 Aug 10;36(32):8516-32. doi: 10.1523/JNEUROSCI.0735-16.2016. J Neurosci. 2016. PMID: 27511021 Free PMC article.
References
- Mantovani A., Sica A., Sozzani S., Allavena P., Vecchi A., Locati M. (2004) Trends Immunol. 25, 677–686 - PubMed
- Martinez F. O., Helming L., Gordon S. (2009) Annu. Rev. Immunol. 27, 451–483 - PubMed
- Minty A., Chalon P., Derocq J. M., Dumont X., Guillemot J. C., Kaghad M., Labit C., Leplatois P., Liauzun P., Miloux B., et al. (1993) Nature 362, 248–250 - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous