Effects of ATPM-ET, a novel κ agonist with partial μ activity, on physical dependence and behavior sensitization in mice - PubMed (original) (raw)
. 2010 Dec;31(12):1547-52.
doi: 10.1038/aps.2010.164. Epub 2010 Nov 22.
Yu-hua WANG, Fu-ying LI, Gang LU, Yi-min TAO, Yun CHENG, Jie CHEN, Xue-jun XU, Zhi-qiang CHI, John L NEUMEYER, Ao ZHANG, Jing-gen LIU
Affiliations
- PMID: 21102484
- PMCID: PMC4002947
- DOI: 10.1038/aps.2010.164
Effects of ATPM-ET, a novel κ agonist with partial μ activity, on physical dependence and behavior sensitization in mice
Jian-feng SUN et al. Acta Pharmacol Sin. 2010 Dec.
Abstract
Aim: to investigate the effects of ATPM-ET [(-)-3-N-Ethylaminothiazolo [5,4-b]-N-cyclopropylmethylmorphinan hydrochloride] on physical dependence and behavioral sensitization to morphine in mice.
Methods: the pharmacological profile of ATPM-ET was characterized using competitive binding and GTPγS binding assays. We then examined the antinociceptive effects of ATPM-ET in the hot plate test. Morphine dependence assay and behavioral sensitization assay were used to determine the effect of ATPM-ET on physical dependence and behavior sensitization to morphine in mice.
Results: the binding assay indicated that ATPM-ET ATPM-ET exhibited a high affinity to both κ- and μ-opioid receptors with K(i) values of 0.15 nmol/L and 4.7 nmol/L, respectively, indicating it was a full κ-opioid receptor agonist and a partial μ-opioid receptor agonist. In the hot plate test, ATPM-ET produced a dose-dependent antinociceptive effect, with an ED(50) value of 2.68 (2.34-3.07) mg/kg. Administration of ATPM-ET (1 and 2 mg/kg, sc) prior to naloxone (3.0 mg/kg, sc) injection significantly inhibited withdrawal jumping of mice. In addition, ATPM-ET (1 and 2 mg/kg, sc) also showed a trend toward decreasing morphine withdrawal-induced weight loss. ATPM-ET (1.5 and 3 mg/kg, sc) 15 min before the morphine challenge significantly inhibited the morphine-induced behavior sensitization (P<0.05).
Conclusion: ATPM-ET may have potential as a therapeutic agent for the treatment of drug abuse.
Figures
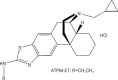
Figure 1
Chemical structure of ATPM-ET.
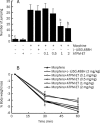
Figure 2
Effects of ATPM-ET and (−)U50,488H on naloxone-precipitated jumping and body weight loss in mice treated chronically with morphine. Mice were treated with progressively increasing doses of either ATPM-ET or morphine alone or treated with progressively increasing doses of morphine concomitantly with ATPM-ET (0.1–2 mg/kg, subcutaneously) or (-)U50,488H (2 mg/kg, subcutaneously) for 5 days. Morphine withdrawal jumping and body weight loss were precipitated by naloxone (3 mg/kg, subcutaneously). (A) The number of jumping events was measured for 30 min after naloxone injection. (B) Body weight loss was measured initially and 30 and 60 min after the naloxone injection. Data were presented as the mean±SEM from 10 animals. b_P_<0.05, c_P_<0.01 vs morphine alone.
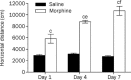
Figure 3
The induction of morphine sensitization in mice. Two groups were given morphine (10 mg/kg, subcutaneously) or saline (10 mL/kg, subcutaneously) for 7 consecutive days and their activity was measured for 1 h after each administration of drug (or saline) on days 1, 4, and 7. Data are shown as means±SEM. (n_=10 per group). b_P<0.05, c_P_<0.01 vs the corresponding saline group. e_P_<0.05, f_P_<0.01 vs the first administration group.
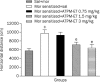
Figure 4
Effect of ATPM-ET on morphine-induced behavioral sensitization. Mice in the control group were given saline, and the other mice received morphine (10 mg/kg, subcutaneously) for 7 consecutive days. After a 7-d washout period, the mice were challenged with morphine (10 mg/kg, subcutaneously) 15 min after an injection of ATPM-ET (0.75, 1.5, 3 mg/kg, subcutaneously). The mice were then put into test chambers to measure their locomotor activity for 1 h. Data are presented as means±SEM (n_=10 per group). c_P<0.01 vs saline group. e_P_<0.05 vs morphine sensitized/saline group.
Similar articles
- The anxiolytic- and antidepressant-like effects of ATPM-ET, a novel κ agonist and μ partial agonist, in mice.
Wang Q, Long Y, Hang A, Zan GY, Shu XH, Wang YJ, Liu JG. Wang Q, et al. Psychopharmacology (Berl). 2016 Jun;233(12):2411-8. doi: 10.1007/s00213-016-4292-z. Epub 2016 Apr 26. Psychopharmacology (Berl). 2016. PMID: 27113225 - Pharmacological characterization of ATPM [(-)-3-aminothiazolo[5,4-b]-N-cyclopropylmethylmorphinan hydrochloride], a novel mixed kappa-agonist and mu-agonist/-antagonist that attenuates morphine antinociceptive tolerance and heroin self-administration behavior.
Wang YJ, Tao YM, Li FY, Wang YH, Xu XJ, Chen J, Cao YL, Chi ZQ, Neumeyer JL, Zhang A, Liu JG. Wang YJ, et al. J Pharmacol Exp Ther. 2009 Apr;329(1):306-13. doi: 10.1124/jpet.108.142802. Epub 2009 Jan 9. J Pharmacol Exp Ther. 2009. PMID: 19136637 Free PMC article. - Pharmacological characterization and therapeutic potential for the treatment of opioid abuse with ATPM-ET, an N-ethyl substituted aminothiazolomorphinan with κ agonist and μ agonist/antagonist activity.
Sun JF, Wang YH, Chai JR, Li FY, Hang A, Lu G, Tao YM, Cheng Y, Chi ZQ, Neumeyer JL, Zhang A, Liu JG, Wang YJ. Sun JF, et al. Eur J Pharmacol. 2014 Oct 5;740:455-63. doi: 10.1016/j.ejphar.2014.06.045. Epub 2014 Jul 3. Eur J Pharmacol. 2014. PMID: 24998879 - Behavioral and molecular evidence for a feedback interaction between morphine and HIV-1 viral proteins.
Chang SL, Connaghan KP. Chang SL, et al. J Neuroimmune Pharmacol. 2012 Jun;7(2):332-40. doi: 10.1007/s11481-011-9324-1. Epub 2011 Nov 15. J Neuroimmune Pharmacol. 2012. PMID: 22083500 Review. - Diels-Alder Adducts of Morphinan-6,8-Dienes and Their Transformations.
Marton J, Fekete A, Cumming P, Hosztafi S, Mikecz P, Henriksen G. Marton J, et al. Molecules. 2022 Apr 30;27(9):2863. doi: 10.3390/molecules27092863. Molecules. 2022. PMID: 35566212 Free PMC article. Review.
Cited by
- Synthesis and pharmacological evaluation of aminothiazolomorphinans at the mu and kappa opioid receptors.
Provencher BA, Sromek AW, Li W, Russell S, Chartoff E, Knapp BI, Bidlack JM, Neumeyer JL. Provencher BA, et al. J Med Chem. 2013 Nov 14;56(21):8872-8. doi: 10.1021/jm401290y. Epub 2013 Oct 29. J Med Chem. 2013. PMID: 24107104 Free PMC article. - Strategies for Developing κ Opioid Receptor Agonists for the Treatment of Pain with Fewer Side Effects.
Paton KF, Atigari DV, Kaska S, Prisinzano T, Kivell BM. Paton KF, et al. J Pharmacol Exp Ther. 2020 Nov;375(2):332-348. doi: 10.1124/jpet.120.000134. Epub 2020 Sep 10. J Pharmacol Exp Ther. 2020. PMID: 32913006 Free PMC article. Review. - Cebranopadol as a Novel Promising Agent for the Treatment of Pain.
Ziemichod W, Kotlinska J, Gibula-Tarlowska E, Karkoszka N, Kedzierska E. Ziemichod W, et al. Molecules. 2022 Jun 21;27(13):3987. doi: 10.3390/molecules27133987. Molecules. 2022. PMID: 35807228 Free PMC article. Review. - Novel κ-opioid receptor agonist MB-1C-OH produces potent analgesia with less depression and sedation.
Zhang LS, Wang J, Chen JC, Tao YM, Wang YH, Xu XJ, Chen J, Xu YG, Xi T, Hu XW, Wang YJ, Liu JG. Zhang LS, et al. Acta Pharmacol Sin. 2015 May;36(5):565-71. doi: 10.1038/aps.2014.145. Epub 2015 Mar 30. Acta Pharmacol Sin. 2015. PMID: 25816912 Free PMC article. - The anxiolytic- and antidepressant-like effects of ATPM-ET, a novel κ agonist and μ partial agonist, in mice.
Wang Q, Long Y, Hang A, Zan GY, Shu XH, Wang YJ, Liu JG. Wang Q, et al. Psychopharmacology (Berl). 2016 Jun;233(12):2411-8. doi: 10.1007/s00213-016-4292-z. Epub 2016 Apr 26. Psychopharmacology (Berl). 2016. PMID: 27113225
References
- Wells JL, Bartlett JL, Ananthan S, Bilsky EJ. In vivo pharmacological characterization of SoRI 9409, a nonpeptidic opioid mu-agonist/delta-antagonist that produces limited antinociceptive tolerance and attenuates morphine physical dependence. J Pharmacol Exp Ther. 2001;297:597–605. - PubMed
- Sepulveda J, Ortega A, Zapata G, Contreras E. Acamprosate decreases the induction of tolerance and physical dependence in morphine-treated mice. Eur J Pharmacol. 2002;445:87–91. - PubMed
- Fattore L, Spano MS, Deiana S, Melis V, Cossu G, Fadda P, et al. An endocannabinoid mechanism in relapse to drug seeking: a review of animal studies and clinical perspectives. Brain Res Rev. 2007;53:1–16. - PubMed
- Lyvers M. Drug addiction as a physical disease: the role of physical dependence and other chronic drug-induced neurophysiological changes in compulsive drug self-administration. Exp Clin Psychopharmacol. 1998;6:107–25. - PubMed
- Bell K, Salmon A. Pain, physical dependence and pseudoaddiction: redefining addiction for 'nice' people. Int J Drug Policy. 2009;20:170–8. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous