Stage-specific sensitivity to p53 restoration during lung cancer progression - PubMed (original) (raw)
Stage-specific sensitivity to p53 restoration during lung cancer progression
David M Feldser et al. Nature. 2010.
Abstract
Tumorigenesis is a multistep process that results from the sequential accumulation of mutations in key oncogene and tumour suppressor pathways. Personalized cancer therapy that is based on targeting these underlying genetic abnormalities presupposes that sustained inactivation of tumour suppressors and activation of oncogenes is essential in advanced cancers. Mutations in the p53 tumour-suppressor pathway are common in human cancer and significant efforts towards pharmaceutical reactivation of defective p53 pathways are underway. Here we show that restoration of p53 in established murine lung tumours leads to significant but incomplete tumour cell loss specifically in malignant adenocarcinomas, but not in adenomas. We define amplification of MAPK signalling as a critical determinant of malignant progression and also a stimulator of Arf tumour-suppressor expression. The response to p53 restoration in this context is critically dependent on the expression of Arf. We propose that p53 not only limits malignant progression by suppressing the acquisition of alterations that lead to tumour progression, but also, in the context of p53 restoration, responds to increased oncogenic signalling to mediate tumour regression. Our observations also underscore that the p53 pathway is not engaged by low levels of oncogene activity that are sufficient for early stages of lung tumour development. These data suggest that restoration of pathways important in tumour progression, as opposed to initiation, may lead to incomplete tumour regression due to the stage-heterogeneity of tumour cell populations.
Figures
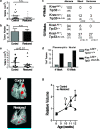
Figure 1. Lung adenomas are apathetic to Trp53 restoration, whereas adenocarcinomas are responsive
a, Individual tumour volumes were determined by μCT two weeks after _Trp53_-restoration that began at four weeks of age. b, Histological sections were evaluated for tumor number in control (n=10) and restored (n=12) mice. c, Tumour number and grade at four and ten weeks of age. Individual tumours from Kras LA2/+;Trp53 LSL/LSL (n=5) and Kras LA2/+;Trp53+/+ (n= 4) mice. The percentage and number (in parentheses) of tumours is indicated. d, Pleomorphic nuclei in tumour samples from c (see also Supplemental Fig. 5). e, Restoration of Trp53 in ten-week old animals results in significantly diminished tumour size. Tumour volumes determined by μCT two weeks after p53 restoration. f, Representative tomograms from μCT analysis at 12 weeks. g, Serial μCT analysis of individual tumours. Average relative size and standard error of tumours are plotted.
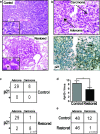
Figure 2. Adenocarcinoma cells are sensitive to Trp53 restoration and are specifically eliminated from lung tumours
a, Histological sections from control and _Trp53_-restored tumours stained with hemotoxylin and eosin (H&E). Inset of _Trp53_-restored tumours show monomorphic nuclei and tumour fissures filled with foam macrophages (asterisks). Scale bars equal 50 microns. b, Tumour sections three days post Trp53 restoration. H&E stained tumour section (top) with adenoma and carcinoma areas indicated. Serial section stained with p21 antibody (bottom). c, Contingency tables showing coincidence of p21 and adenocarcinoma 3 days post Trp53 restoration. d, Average number and standard deviation of pH3 positive cells/μm2 for each tumor are shown. Trp53 restoration leads to significantly fewer mitoses. e, Tumour grades in control and Trp53 restored animals two weeks after initial treatment (Vehicle treated tumours n= 60, tamoxifen treated tumours n=47).
Figure 3. Adenocarcinomas are typified by amplified MAPK signaling
a, Serial tumour sections of a mixed grade tumour stained with (i) H&E, (ii) anti-p-Mek, or (iii) anti-p-Erk. Dashed lines outline high-grade (blue) and low-grade (black) areas. b, Contingency tables representing number of tumours with high or low MAPK signaling, and the corresponding tumour grades in Kras LA2/+;Trp53+/+ and Kras LA2/+;Trp53 LSL/LSL at the four and ten-week time points. c, A subset of tissue sections from a were stained with BAC probes surrounding the Kras locus on mouse chromosome 6. Kras signals (red) and DAPI/DNA (blue) counter stain are shown in an adenoma and a carcinoma. Asterisks indicate nuclei with greater than two Kras signals. d, Contingency plot of tumours with high p-Erk staining shows significantly fewer tumours with high p-Erk staining after Trp53 restoration.
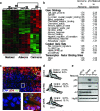
Figure 4. Arf is specifically expressed in adenocarcinomas and sensitizes lung cancer cells to Trp53 restoration
a, Hierarchical clustering of samples based on the Adenocarcinoma Signature. Confidence values (percent) are indicated at the top of major clades. b, GSEA analysis. Notable gene sets are listed with normalized enrichment scores for each comparison. Positive and negative enrichment scores indicate correlation and anti-correlation respectively. c, Tissue sections from Kras LA2/+;Trp53 LSL/LSL adenomas, mixed-grade tumors, and adenocarcinomas were co-labeled with antibodies to p-Erk (red) and Arf (green). Six adjacent fields of view of a mixed-grade tumour (top), and deconvoluted z-stack images (bottom) of dashed regions of an adenoma area (left) and adenocarcinoma area (right) are shown. d, Proliferation assay in adenocarcinoma-derived cell lines. Knockdown with retrovirally expressed shRNAs to Trp53 or Cdkn2a (Arf-specific transcript), or control cells 48-hours post Trp53 restoration. Percentage of BrdU+ cells is indicated. e, Adenocarcinoma cell lysates from d were subjected to immunoblot analysis for p53, p21, Arf, and Hsp90 (loading control).
Comment in
- Cancer: The blind spot of p53.
Berns A. Berns A. Nature. 2010 Nov 25;468(7323):519-20. doi: 10.1038/468519a. Nature. 2010. PMID: 21107421 No abstract available. - Tumour suppressors: Selective justice.
McCarthy N. McCarthy N. Nat Rev Cancer. 2011 Jan;11(1):4. doi: 10.1038/nrc2987. Nat Rev Cancer. 2011. PMID: 21213953 No abstract available.
Similar articles
- Selective activation of p53-mediated tumour suppression in high-grade tumours.
Junttila MR, Karnezis AN, Garcia D, Madriles F, Kortlever RM, Rostker F, Brown Swigart L, Pham DM, Seo Y, Evan GI, Martins CP. Junttila MR, et al. Nature. 2010 Nov 25;468(7323):567-71. doi: 10.1038/nature09526. Nature. 2010. PMID: 21107427 Free PMC article. - Requirement for NF-kappaB signalling in a mouse model of lung adenocarcinoma.
Meylan E, Dooley AL, Feldser DM, Shen L, Turk E, Ouyang C, Jacks T. Meylan E, et al. Nature. 2009 Nov 5;462(7269):104-7. doi: 10.1038/nature08462. Epub 2009 Oct 21. Nature. 2009. PMID: 19847165 Free PMC article. - CKIα ablation highlights a critical role for p53 in invasiveness control.
Elyada E, Pribluda A, Goldstein RE, Morgenstern Y, Brachya G, Cojocaru G, Snir-Alkalay I, Burstain I, Haffner-Krausz R, Jung S, Wiener Z, Alitalo K, Oren M, Pikarsky E, Ben-Neriah Y. Elyada E, et al. Nature. 2011 Feb 17;470(7334):409-13. doi: 10.1038/nature09673. Nature. 2011. PMID: 21331045 - Molecular pathogenesis of transplacentally induced mouse lung tumors.
Miller MS, Leone-Kabler S, Rollins LA, Wessner LL, Fan M, Schaeffer DO, McEntee MF, O'Sullivan MG. Miller MS, et al. Exp Lung Res. 1998 Jul-Aug;24(4):557-77. doi: 10.3109/01902149809087386. Exp Lung Res. 1998. PMID: 9659583 Review. - Activation of p53 by oncogenes.
Lowe SW. Lowe SW. Endocr Relat Cancer. 1999 Mar;6(1):45-8. doi: 10.1677/erc.0.0060045. Endocr Relat Cancer. 1999. PMID: 10732786 Review.
Cited by
- Recent discoveries in the cycling, growing and aging of the p53 field.
McCubrey JA, Demidenko ZN. McCubrey JA, et al. Aging (Albany NY). 2012 Dec;4(12):887-93. doi: 10.18632/aging.100529. Aging (Albany NY). 2012. PMID: 23425920 Free PMC article. Review. - Global genomic profiling reveals an extensive p53-regulated autophagy program contributing to key p53 responses.
Kenzelmann Broz D, Spano Mello S, Bieging KT, Jiang D, Dusek RL, Brady CA, Sidow A, Attardi LD. Kenzelmann Broz D, et al. Genes Dev. 2013 May 1;27(9):1016-31. doi: 10.1101/gad.212282.112. Genes Dev. 2013. PMID: 23651856 Free PMC article. - How do K-RAS-activated cells evade cellular defense mechanisms?
Lee YS, Bae SC. Lee YS, et al. Oncogene. 2016 Feb 18;35(7):827-32. doi: 10.1038/onc.2015.153. Epub 2015 May 11. Oncogene. 2016. PMID: 25961920 Free PMC article. Review. - Structure and Function of p53-DNA Complexes with Inactivation and Rescue Mutations: A Molecular Dynamics Simulation Study.
Kamaraj B, Bogaerts A. Kamaraj B, et al. PLoS One. 2015 Aug 5;10(8):e0134638. doi: 10.1371/journal.pone.0134638. eCollection 2015. PLoS One. 2015. PMID: 26244575 Free PMC article. - NKX2-1-mediated p53 expression modulates lung adenocarcinoma progression via modulating IKKβ/NF-κB activation.
Chen PM, Wu TC, Cheng YW, Chen CY, Lee H. Chen PM, et al. Oncotarget. 2015 Jun 10;6(16):14274-89. doi: 10.18632/oncotarget.3695. Oncotarget. 2015. PMID: 25881545 Free PMC article.
References
- Hanahan D, Weinberg RA. The hallmarks of cancer. Cell. 2000;100:57–70. - PubMed
- Selivanova G, Wiman KG. Reactivation of mutant p53: molecular mechanisms and therapeutic potential. Oncogene. 2007;26:2243–2254. - PubMed
- Wang W, El-Deiry WS. Restoration of p53 to limit tumor growth. Curr Opin Oncol. 2008;20:90–96. - PubMed
- Martins CP, Brown-Swigart L, Evan GI. Modeling the therapeutic efficacy of p53 restoration in tumors. Cell. 2006;127:1323–1334. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- P30-CA14051/CA/NCI NIH HHS/United States
- P30 CA014051-37/CA/NCI NIH HHS/United States
- P30 CA014051-39/CA/NCI NIH HHS/United States
- P30 CA014051-38/CA/NCI NIH HHS/United States
- HHMI_/Howard Hughes Medical Institute/United States
- P30 CA014051/CA/NCI NIH HHS/United States
- P30 CA014051-40/CA/NCI NIH HHS/United States
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
Research Materials
Miscellaneous