Phase II study of bevacizumab plus temozolomide during and after radiation therapy for patients with newly diagnosed glioblastoma multiforme - PubMed (original) (raw)
Clinical Trial
. 2011 Jan 10;29(2):142-8.
doi: 10.1200/JCO.2010.30.2729. Epub 2010 Dec 6.
Anh Tran, Phioanh L Nghiemphu, Whitney B Pope, Orestes E Solis, Michael Selch, Emese Filka, William H Yong, Paul S Mischel, Linda M Liau, Surasak Phuphanich, Keith Black, Scott Peak, Richard M Green, Cynthia E Spier, Tatjana Kolevska, Jonathan Polikoff, Louis Fehrenbacher, Robert Elashoff, Timothy Cloughesy
Affiliations
- PMID: 21135282
- PMCID: PMC3058273
- DOI: 10.1200/JCO.2010.30.2729
Clinical Trial
Phase II study of bevacizumab plus temozolomide during and after radiation therapy for patients with newly diagnosed glioblastoma multiforme
Albert Lai et al. J Clin Oncol. 2011.
Abstract
Purpose: This open-label, prospective, multicenter single-arm phase II study combined bevacizumab (BV) with radiation therapy (RT) and temozolomide (TMZ) for the treatment of newly diagnosed glioblastoma (GBM). The objectives were to determine the efficacy of this treatment combination and the associated toxicity.
Patients and methods: Seventy patients with newly diagnosed GBM were enrolled between August 2006 and November 2008. Patients received standard RT starting within 3 to 6 weeks after surgery with concurrent administration of daily TMZ and biweekly BV. After completion of RT, patients resumed TMZ for 5 days every 4 weeks and continued biweekly BV. MGMT promoter methylation was assessed on patient tumor tissue. A University of California, Los Angeles/Kaiser Permanente Los Angeles (KPLA) control cohort of newly diagnosed patients treated with first-line RT and TMZ who had mostly received BV at recurrence was derived for comparison.
Results: The overall survival (OS) and progression-free survival (PFS) were 19.6 and 13.6 months, respectively, compared to 21.1 and 7.6 months in the University of California, Los Angeles/KPLA control cohort, and 14.6 and 6.9 months in the European Organisation for Research and Treatment of Cancer-National Cancer Institute of Canada cohort. Correlation of MGMT promoter methylation and improved OS and PFS was retained in the study group. Comparative subset analysis showed that poor prognosis patients (recursive partitioning analysis class V/VI) may derive an early benefit from the use of first-line BV. Toxicity attributable to RT/TMZ was similar, and additional toxicities were consistent with those reported in other BV trials.
Conclusion: Patients treated with BV and TMZ during and after RT showed improved PFS without improved OS compared to the University of California, Los Angeles/KPLA control group. Additional studies are warranted to determine if BV administered first-line improves survival compared to BV at recurrence.
Conflict of interest statement
Authors' disclosures of potential conflicts of interest and author contributions are found at the end of this article.
Figures
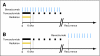
Fig 1.
Treatment schema for (A) study group and (B) University of California, Los Angeles/Kaiser Permanente Los Angeles control group in which most patients received bevacizumab at recurrence. Wks, weeks.
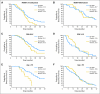
Fig 2.
Kaplan-Meier analysis of (A) overall survival and (B) progression-free survival comparing current study group (gold; radiation therapy [RT] + temozolomide [TMZ] + bevacizumab [BV]) with University of California, Los Angeles/Kaiser Permanente Los Angeles control group (blue; RT + TMZ). Use of first-line BV shows early benefit in progression-free survival [Fleming(1,0) weighted log-rank test P < .005] and trended toward worse overall survival with later follow-up [Fleming(0,1) weighted log-rank test P < .06].
Fig 3.
Prespecified Kaplan-Meier analysis of overall survival comparing current study (gold) and University of California, Los Angeles/ Kaiser Permanente Los Angeles (KPLA) control (blue) groups for (A,B) MGMT methylation, (C,D) recursive partitioning analysis (RPA) class, and (E,F) age. (A) Unmethylated MGMT promoter subgroup appears to do less well with the addition of bevacizumab (BV) in first-line treatment [(Fleming(0,1); P < .005], while showing no difference within the (B) methylated subgroup [Fleming (1,0); P = .83). (D) RPA V/VI group shows early benefit from the treatment compared with University of California, Los Angeles/KPLA control [Fleming(2,0) P < .05), while showing no difference within the RPA III/IV group (C) [Fleming(0,1) P = .10]. (E) Patients with age younger than 50 years did significantly less well with BV compared to the University of California, Los Angeles/KPLA control group (log-rank P < .005), without any difference in the (F) older than 50 years group [Fleming(1,0); P = .42]. RT, radiation therapy; TMZ, temozolomide.
Fig A1.
Recursive partitioning analysis (RPA) classification of current study group and University of California, Los Angeles/KPLA control group. UCLA, University of California, Los Angeles; KPLA, Kaiser Permanente, Los Angeles; pts, patients; KPS, Karnofsky performance status; NFS, neurological functional status; MS, mental status.
Fig A2.
Progression-free survival and overall survival by Kaplan-Meier analysis of current study group stratified by MGMT promoter methylation status (gold, methylated; blue, unmethylated). Methylated MGMT promoter group responded significantly better than unmethylated group (progression-free survival and overall survival P < .005).
Comment in
- Taming glioblastoma by targeting angiogenesis: 3 years later.
Wong ET, Brem S. Wong ET, et al. J Clin Oncol. 2011 Jan 10;29(2):124-6. doi: 10.1200/JCO.2010.32.5282. Epub 2010 Dec 6. J Clin Oncol. 2011. PMID: 21135277 No abstract available.
Similar articles
- Phase II pilot study of bevacizumab in combination with temozolomide and regional radiation therapy for up-front treatment of patients with newly diagnosed glioblastoma multiforme: interim analysis of safety and tolerability.
Lai A, Filka E, McGibbon B, Nghiemphu PL, Graham C, Yong WH, Mischel P, Liau LM, Bergsneider M, Pope W, Selch M, Cloughesy T. Lai A, et al. Int J Radiat Oncol Biol Phys. 2008 Aug 1;71(5):1372-80. doi: 10.1016/j.ijrobp.2007.11.068. Epub 2008 Mar 20. Int J Radiat Oncol Biol Phys. 2008. PMID: 18355978 Clinical Trial. - Dexamethasone administration during definitive radiation and temozolomide renders a poor prognosis in a retrospective analysis of newly diagnosed glioblastoma patients.
Shields LB, Shelton BJ, Shearer AJ, Chen L, Sun DA, Parsons S, Bourne TD, LaRocca R, Spalding AC. Shields LB, et al. Radiat Oncol. 2015 Oct 31;10:222. doi: 10.1186/s13014-015-0527-0. Radiat Oncol. 2015. PMID: 26520780 Free PMC article. - Survival benefits of hypofractionated radiotherapy combined with temozolomide or temozolomide plus bevacizumab in elderly patients with glioblastoma aged ≥ 75 years.
Ohno M, Miyakita Y, Takahashi M, Igaki H, Matsushita Y, Ichimura K, Narita Y. Ohno M, et al. Radiat Oncol. 2019 Nov 12;14(1):200. doi: 10.1186/s13014-019-1389-7. Radiat Oncol. 2019. PMID: 31718669 Free PMC article. - The effectiveness and cost-effectiveness of carmustine implants and temozolomide for the treatment of newly diagnosed high-grade glioma: a systematic review and economic evaluation.
Garside R, Pitt M, Anderson R, Rogers G, Dyer M, Mealing S, Somerville M, Price A, Stein K. Garside R, et al. Health Technol Assess. 2007 Nov;11(45):iii-iv, ix-221. doi: 10.3310/hta11450. Health Technol Assess. 2007. PMID: 17999840 Review.
Cited by
- The future of high-grade glioma: Where we are and where are we going.
Le Rhun E, Rhun EL, Taillibert S, Chamberlain MC. Le Rhun E, et al. Surg Neurol Int. 2015 Feb 13;6(Suppl 1):S9-S44. doi: 10.4103/2152-7806.151331. eCollection 2015. Surg Neurol Int. 2015. PMID: 25722939 Free PMC article. - The evolution of the EGFRvIII (rindopepimut) immunotherapy for glioblastoma multiforme patients.
Paff M, Alexandru-Abrams D, Hsu FP, Bota DA. Paff M, et al. Hum Vaccin Immunother. 2014;10(11):3322-31. doi: 10.4161/21645515.2014.983002. Hum Vaccin Immunother. 2014. PMID: 25625931 Free PMC article. Review. - Towards personalized therapy for patients with glioblastoma.
Shirai K, Chakravarti A. Shirai K, et al. Expert Rev Anticancer Ther. 2011 Dec;11(12):1935-44. doi: 10.1586/era.11.103. Expert Rev Anticancer Ther. 2011. PMID: 22117160 Free PMC article. Review. - Using lithium as a neuroprotective agent in patients with cancer.
Khasraw M, Ashley D, Wheeler G, Berk M. Khasraw M, et al. BMC Med. 2012 Nov 2;10:131. doi: 10.1186/1741-7015-10-131. BMC Med. 2012. PMID: 23121766 Free PMC article. Review. - Glutamine uptake and utilization of human mesenchymal glioblastoma in orthotopic mouse model.
Oizel K, Yang C, Renoult O, Gautier F, Do QN, Joalland N, Gao X, Ko B, Vallette F, Ge WP, Paris F, DeBerardinis RJ, Pecqueur C. Oizel K, et al. Cancer Metab. 2020 Aug 10;8:9. doi: 10.1186/s40170-020-00215-8. eCollection 2020. Cancer Metab. 2020. PMID: 32789014 Free PMC article.
References
- Stupp R, Hegi ME, Mason WP, et al. Effects of radiotherapy with concomitant and adjuvant temozolomide versus radiotherapy alone on survival in glioblastoma in a randomised phase III study: 5-year analysis of the EORTC-NCIC trial. The Lancet Oncology. 2009;10:459–466. - PubMed
- Curran WJ, Jr, Scott CB, Horton J, et al. Recursive partitioning analysis of prognostic factors in three Radiation Therapy Oncology Group malignant glioma trials. J Natl Cancer Inst. 1993;85:704–710. - PubMed
- Friedman HS, Prados MD, Wen PY, et al. Bevacizumab alone and in combination with irinotecan in recurrent glioblastoma. J Clin Oncol. 2009;27:4733–4740. - PubMed
- Chen HX, Cleck JN. Adverse effects of anticancer agents that target the VEGF pathway. Nat Rev Clin Oncol. 2009;6:465–477. - PubMed
- Stupp R, Mason WP, van den Bent MJ, et al. Radiotherapy plus concomitant and adjuvant temozolomide for glioblastoma. N Engl J Med. 2005;352:987–996. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials