Overexpression of glutaminyl cyclase, the enzyme responsible for pyroglutamate A{beta} formation, induces behavioral deficits, and glutaminyl cyclase knock-out rescues the behavioral phenotype in 5XFAD mice - PubMed (original) (raw)
Overexpression of glutaminyl cyclase, the enzyme responsible for pyroglutamate A{beta} formation, induces behavioral deficits, and glutaminyl cyclase knock-out rescues the behavioral phenotype in 5XFAD mice
Sadim Jawhar et al. J Biol Chem. 2011.
Abstract
Pyroglutamate-modified Aβ (AβpE3-42) peptides are gaining considerable attention as potential key players in the pathology of Alzheimer disease (AD) due to their abundance in AD brain, high aggregation propensity, stability, and cellular toxicity. Overexpressing AβpE3-42 induced a severe neuron loss and neurological phenotype in TBA2 mice. In vitro and in vivo experiments have recently proven that the enzyme glutaminyl cyclase (QC) catalyzes the formation of AβpE3-42. The aim of the present work was to analyze the role of QC in an AD mouse model with abundant AβpE3-42 formation. 5XFAD mice were crossed with transgenic mice expressing human QC (hQC) under the control of the Thy1 promoter. 5XFAD/hQC bigenic mice showed significant elevation in TBS, SDS, and formic acid-soluble AβpE3-42 peptides and aggregation in plaques. In 6-month-old 5XFAD/hQC mice, a significant motor and working memory impairment developed compared with 5XFAD. The contribution of endogenous QC was studied by generating 5XFAD/QC-KO mice (mouse QC knock-out). 5XFAD/QC-KO mice showed a significant rescue of the wild-type mice behavioral phenotype, demonstrating the important contribution of endogenous mouse QC and transgenic overexpressed QC. These data clearly demonstrate that QC is crucial for modulating AβpE3-42 levels in vivo and prove on a genetic base the concept that reduction of QC activity is a promising new therapeutic approach for AD.
Figures
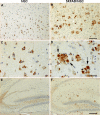
FIGURE 1.
Immunohistochemical staining of hQC in hQC and 5XFAD/hQC mice. Expression of human transgenic QC was detected in pyramidal neurons in the cortex of hQC (A and C) and 5XFAD/hQC mice and in plaque-associated dystrophic neurites (arrows) of 5XFAD/hQC mice (B and D). In addition, hQC staining was detected in mossy fibers of the hippocampal formation of hQC and 5XFAD/hQC mice (E and F). Scale bars, 100 μm (A and B), 50 μm (C and D), 200 μm (E and F).
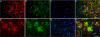
FIGURE 2.
Transgene human QC is co-localized with APP. Double immunostaining in the cortex of 5XFAD/hQC mice using antibodies against APP (red; A and E), QC (green; B and F) and DAPI (blue; C and G). APP and QC showed co-localization in dystrophic neurites of plaques and in the somatodendritic compartment of pyramidal neurons in the merged images (yellow; D, H, inset in H). Scale bars, 50 μm (A–D), 20 μm (E–H).
FIGURE 3.
Effect of hQC overexpression and QC knock-out on plaque load in 5XFAD mice. A, plaque staining in the cortex using antibodies against generic Aβ (NT78) and pyroglutamate-modified Aβ (2–48) in 5XFAD, 5XFAD/hQC, and 5XFAD/QC-KO mice. B, quantification of plaque load demonstrating significantly elevated Aβ and AβpE3 levels in 5XFAD/hQC and significantly reduced levels in 5XFAD/QC-KO mouse brain. Scale bar, 200 μm. *, p < 0.05; **, p < 0.01. Error bars, S.E.
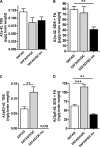
FIGURE 4.
Effect of hQC overexpression and QC knock-out on Aβ levels in 5XFAD mice. Quantification of Aβx-42 and AβpE3–42 using ELISA showed significant changes in TBS, SDS, and formic acid (FA) fractions in 5XFAD, 5XFAD/hQC, and 5XFAD/QC-KO mouse brain. SDS and FA fractions were pooled for quantification. Aβx-42 levels were significantly reduced in the SDS+FA fraction of 5XFAD/QC-KO mice. AβpE3–42 levels were significantly elevated in all fractions in 5XFAD/hQC mice. Although in the TBS fraction of 5XFAD/QC-KO mice the levels of AβpE3–42 were below the limit of quantitation, in the SDS+FA fractions of 5XFAD/QC-KO mice the levels of AβpE3–42 were significantly reduced. **, p < 0.01; ***, p < 0.001. LOQ, limit of quantitation. Error bars, S.E.
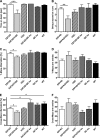
FIGURE 5.
Effect of hQC overexpression and QC knock-out on behavioral performance in 5XFAD mice. A and B, 5XFAD/hQC mice showed a significantly reduced motor performance in balance beam (A) and string suspension task (B) compared with 5XFAD mice. C, in addition, working memory deficits were detected in 5XFAD/hQC compared with 5XFAD mice using Y- and cross-maze (E). Interestingly, the 5XFAD/QC-KO mice showed a rescue of working memory deficits with alternation frequencies indistinguishable from wild-type mice. D and F, the number of arm entries in Y- and cross-maze did not differ among the groups. *, p < 0.05; **, p < 0.01; ***, p < 0.001.
Similar articles
- Immunohistochemical Evidence from APP-Transgenic Mice for Glutaminyl Cyclase as Drug Target to Diminish pE-Abeta Formation.
Hartlage-Rübsamen M, Bluhm A, Piechotta A, Linnert M, Rahfeld JU, Demuth HU, Lues I, Kuhn PH, Lichtenthaler SF, Roßner S, Höfling C. Hartlage-Rübsamen M, et al. Molecules. 2018 Apr 17;23(4):924. doi: 10.3390/molecules23040924. Molecules. 2018. PMID: 29673150 Free PMC article. - Glutaminyl cyclase contributes to the formation of focal and diffuse pyroglutamate (pGlu)-Aβ deposits in hippocampus via distinct cellular mechanisms.
Hartlage-Rübsamen M, Morawski M, Waniek A, Jäger C, Zeitschel U, Koch B, Cynis H, Schilling S, Schliebs R, Demuth HU, Rossner S. Hartlage-Rübsamen M, et al. Acta Neuropathol. 2011 Jun;121(6):705-19. doi: 10.1007/s00401-011-0806-2. Epub 2011 Feb 8. Acta Neuropathol. 2011. PMID: 21301857 Free PMC article. - Pyroglutamate amyloid β (Aβ) aggravates behavioral deficits in transgenic amyloid mouse model for Alzheimer disease.
Wittnam JL, Portelius E, Zetterberg H, Gustavsson MK, Schilling S, Koch B, Demuth HU, Blennow K, Wirths O, Bayer TA. Wittnam JL, et al. J Biol Chem. 2012 Mar 9;287(11):8154-62. doi: 10.1074/jbc.M111.308601. Epub 2012 Jan 20. J Biol Chem. 2012. PMID: 22267726 Free PMC article. - Pyroglutamate amyloid-β (Aβ): a hatchet man in Alzheimer disease.
Jawhar S, Wirths O, Bayer TA. Jawhar S, et al. J Biol Chem. 2011 Nov 11;286(45):38825-32. doi: 10.1074/jbc.R111.288308. Epub 2011 Sep 29. J Biol Chem. 2011. PMID: 21965666 Free PMC article. Review. - An overview of glutaminyl cyclase inhibitors for Alzheimer's disease.
Coimbra JR, Sobral PJ, Santos AE, Moreira PI, Salvador JA. Coimbra JR, et al. Future Med Chem. 2019 Dec;11(24):3179-3194. doi: 10.4155/fmc-2019-0163. Future Med Chem. 2019. PMID: 31838899 Review.
Cited by
- Development and evolution of human glutaminyl cyclase inhibitors (QCIs): an alternative promising approach for disease-modifying treatment of Alzheimer's disease.
Chen D, Chen Q, Qin X, Tong P, Peng L, Zhang T, Xia C. Chen D, et al. Front Aging Neurosci. 2023 Aug 3;15:1209863. doi: 10.3389/fnagi.2023.1209863. eCollection 2023. Front Aging Neurosci. 2023. PMID: 37600512 Free PMC article. Review. - Neurologic and motor dysfunctions in APP transgenic mice.
Lalonde R, Fukuchi K, Strazielle C. Lalonde R, et al. Rev Neurosci. 2012;23(4):363-79. doi: 10.1515/revneuro-2012-0041. Rev Neurosci. 2012. PMID: 23089603 Free PMC article. Review. - Axonal degeneration in Alzheimer's disease: when signaling abnormalities meet the axonal transport system.
Kanaan NM, Pigino GF, Brady ST, Lazarov O, Binder LI, Morfini GA. Kanaan NM, et al. Exp Neurol. 2013 Aug;246:44-53. doi: 10.1016/j.expneurol.2012.06.003. Epub 2012 Jun 19. Exp Neurol. 2013. PMID: 22721767 Free PMC article. Review. - Recent rodent models for Alzheimer's disease: clinical implications and basic research.
Braidy N, Muñoz P, Palacios AG, Castellano-Gonzalez G, Inestrosa NC, Chung RS, Sachdev P, Guillemin GJ. Braidy N, et al. J Neural Transm (Vienna). 2012 Feb;119(2):173-95. doi: 10.1007/s00702-011-0731-5. Epub 2011 Nov 16. J Neural Transm (Vienna). 2012. PMID: 22086139 Review. - Disturbed Ca2+ homeostasis increases glutaminyl cyclase expression; connecting two early pathogenic events in Alzheimer's disease in vitro.
De Kimpe L, Bennis A, Zwart R, van Haastert ES, Hoozemans JJ, Scheper W. De Kimpe L, et al. PLoS One. 2012;7(9):e44674. doi: 10.1371/journal.pone.0044674. Epub 2012 Sep 7. PLoS One. 2012. PMID: 22970285 Free PMC article.
References
- Selkoe D. J. (1998) Trends Cell Biol. 8, 447–453 - PubMed
- Selkoe D. J., Abraham C. R., Podlisny M. B., Duffy L. K. (1986) J. Neurochem. 46, 1820–1834 - PubMed
- Gorevic P. D., Goñi F., Pons-Estel B., Alvarez F., Peress N. S., Frangione B. (1986) J. Neuropathol. Exp. Neurol. 45, 647–664 - PubMed
- Mori H., Takio K., Ogawara M., Selkoe D. J. (1992) J. Biol. Chem. 267, 17082–17086 - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
Research Materials
Miscellaneous