Advanced glycation end products induce the expression of interleukin-6 and interleukin-8 by receptor for advanced glycation end product-mediated activation of mitogen-activated protein kinases and nuclear factor-κB in human osteoarthritis chondrocytes - PubMed (original) (raw)
Advanced glycation end products induce the expression of interleukin-6 and interleukin-8 by receptor for advanced glycation end product-mediated activation of mitogen-activated protein kinases and nuclear factor-κB in human osteoarthritis chondrocytes
Zafar Rasheed et al. Rheumatology (Oxford). 2011 May.
Abstract
Objective: To investigate whether advanced glycation end products (AGEs) induce the expression of IL-6 and IL-8 through the receptor for AGEs (RAGE)-activated pathways in human OA chondrocytes.
Methods: OA chondrocytes were stimulated with AGE-modified BSA (AGE-BSA). Gene expression of IL-6 and IL-8 was quantified by TaqMan assays and the production was determined using ELISAs. Immunoblotting was used to analyse the activation of mitogen-activated protein kinases (MAPKs) and the degradation of IκBα. Activation of NF-κB was determined using an ELISA. Pharmacological studies to elucidate the involved pathways were executed using transfection with small interfering RNAs (siRNAs), inhibitors of MAPKs and NF-κB.
Results: AGE-BSA induced the expression of IL-6 and IL-8 in OA chondrocytes, which was inhibited by pre-treatment with soluble RAGE (sRAGE) or RAGE knockdown by siRNAs. Treatment with SB202190 (p38-MAPK inhibitor) or PD98059 (ERK inhibitor) inhibited AGE-BSA-induced IL-6 and IL-8 expression. However, SP600125 (JNK inhibitor) had no effect on AGE-BSA-induced IL-6 expression but inhibited the expression of IL-8. Treatment with NF-κB inhibitors suppressed AGE-BSA-induced IL-6 and IL-8 expression.
Conclusions: This is the first study to demonstrate that AGEs induce the expression of IL-6 and IL-8 in OA chondrocytes. A novel finding of our studies is that in OA chondrocytes, AGE-BSA-induced expression of IL-6, but not of IL-8, was independent of the JNK pathway. Activation of NF-κB was an absolute requirement for both IL-6 and IL-8 expression. These results demonstrate that AGE-BSA-induced expression of IL-6 and IL-8 via RAGE is mediated through different MAPK signalling pathways in OA and possibly in other degenerative diseases.
Figures
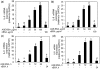
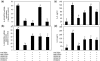
Fig. 1
Expression of IL-6 and IL-8 in AGE-BSA-stimulated primary human OA chondrocytes. Effect of AGE-BSA on the gene expression of IL-6 (a) and IL-8 (b) in primary human OA chondrocytes. Primary human OA chondrocytes were treated with AGE-BSA (5–100 µg/ml) and native BSA (100 µg/ml) for 24 h. Time-dependent effect of AGE-BSA on the gene expression of IL-6 (c) and IL-8 (d) in human OA chondrocytes. Primary chondrocytes were treated with AGE-BSA (100 µg/ml) and native BSA (100 µg/ml) for 0–24 h. Expression of IL-6 or IL-8 mRNA was determined by real-time qRT–PCR using comparative ΔΔ_C_T method. Incubation with native BSA (nBSA) was used as control. Results are representative [mean (
s.e.m
.)] of duplicate experiments with OA chondrocytes obtained from five age- and sex-matched OA donors and differ without a common letter; P < 0.05.
Fig. 2
Enhanced production of IL-6 and IL-8 by AGE-BSA-stimulated primary human OA chondrocytes. Effect of AGE-BSA on the protein production of IL-6 (a) and IL-8 (b) in primary human OA chondrocytes. Primary human OA chondrocytes were treated with AGE-BSA (5–100 µg/ml) and native BSA (100 µg/ml) for 24 h. Kinetics of AGE-BSA-induced production of IL-6 (c) and IL-8 (d) in primary human OA chondrocytes. Primary human OA chondrocytes were treated with AGE-BSA (100 µg/ml) and native BSA (100 µg/ml) for 0–24 h. Production of IL-6 or IL-8 was determined by a sandwich ELISA. Native BSA (nBSA) was used as control. Results are representative [mean (
s.e.m
)] of duplicate experiments with OA chondrocytes obtained from five age- and sex-matched OA donors and differ without a common letter; P < 0.05.
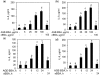
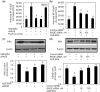
Fig. 3
Effect of sRAGE or RAGE-knockdown on the AGE-BSA or S100A4 protein-induced expression of IL-6 and IL-8 in primary human OA chondrocytes. Primary OA chondrocytes were pre-treated with sRAGE (200 µg/ml) for 2 h and then stimulated with AGE-BSA (50 µg/ml) or S100A4 protein (25 ng/ml) or IL-1β (20 ng/ml) for 24 h. Human OA chondrocytes were transfected with RAGE-siRNA or control siRNA and then stimulated with AGE-BSA for 24 h. Expression of IL-6 (a) and IL-8 (b) mRNA was determined by real-time qPCR and normalized to GAPDH and compared with the levels present in untreated chondrocytes using a comparative ΔΔ_C_T method. Production of IL-6 (c) and IL-8 (d) in the culture medium was quantified by IL-6- or IL-8-specific sandwich ELISA. Results are representative [mean (
s.e.m
)] of duplicate experiments with human OA chondrocytes obtained from two age- and sex-matched OA donors and differ without a common letter; P < 0.05.
Fig. 4
AGE-BSA or S100A4 enhances the production of IL-6 and IL-8 in human OA cartilage explants. Human OA cartilage explants in serum-free DMEM were stimulated with AGE-BSA or S100A4 protein for 24 h at 37°C. Production of IL-6 (a, b) and IL-8 (c, d) in the culture medium was quantified by IL-6- or IL-8-specific sandwich ELISA. Results are representative [mean (
s.e.m
)] of duplicate experiments with human OA chondrocytes obtained from two age- and sex-matched OA donors and differ without a common letter; P < 0.05.
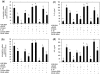
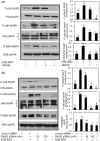
Fig. 5
Activation of RAGE-mediated MAPK signalling by AGE-BSA in primary human OA chondrocytes. Effect of sRAGE (a) and RAGE knockdown (b) on MAPK phosphorylation in AGE-BSA-stimulated OA chondrocytes. After pre-treatment with sRAGE (200 µg/ml) for 2 h at 37°C or RAGE-siRNA (30–100 nM) transfection, human OA chondrocytes (70–80% confluent) were incubated with AGE-BSA (50–100 µg/ml) for 45 min, then the phosphorylation (P) of p38-MAPK, JNK and ERK was determined by western immunoblot analysis. Band images were digitally captured and the band intensities were obtained using UN-San-It software and are expressed in average pixels. Data shown are cumulative of two experiments. Average pixel values presented as mean (
s.e.m
); data without a common letter differ; P < 0.05.
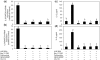
Fig. 6
Different MAPKs regulate AGE-BSA-induced expression of IL-6 and IL-8 in primary human OA chondrocytes. Effect of MAPK inhibition on the gene expression of IL-6 (a) and IL-8 (b) in AGE-BSA-stimulated OA chondrocytes. Primary human OA chondrocytes were pre-treated with inhibitors of p38 (SB202190), JNK (SP600125) and ERK (PD98059) MAPKs for 1 h and then stimulated with AGE-BSA (100 µg/ml) for 24 h. Expression of IL-6 or IL-8 mRNA was determined by real-time qPCR and normalized to GAPDH and compared with the levels present in control using the comparative ΔΔ_C_T method. Effect of MAPK inhibition on the protein production of IL-6 (c) and IL-8 (d) in culture medium of AGE-BSA-stimulated OA chondrocytes. Primary human OA chondrocytes were pre-treated with MAPK inhibitors SB202190, SP600125, PD98059 for 1 h and then stimulated with AGE-BSA (100 µg/ml) for 24 h. Production of IL-6 or IL-8 was quantified by a sandwich ELISA. Primary OA chondrocytes incubated with native BSA (100 µg/ml) were used as control. The concentration of SB202190, SP600125 and PD98059 used in these studies was 100, 10 and 50 µM, respectively. Results are representative [mean (
s.e.m
)] of duplicate experiments with OA chondrocytes obtained from five age- and sex-matched OA donors and differ without a common letter; P < 0.05.
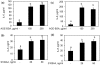
Fig. 7
Activation of RAGE-mediated NF-κB signalling by AGE-BSA in primary human OA chondrocytes. (a) Primary chondrocytes were pre-treated with sRAGE (200 µg/ml) for 2 h prior to AGE-BSA (50 µg/ml) stimulation or (b) RAGE-siRNA-transfected OA chondrocytes were stimulated with AGE-BSA (100 µg/ml) for 45 min and NF-κB p65 was determined in cell extracts (3 µg) by highly sensitive and specific ELISA. TNF-α-treated HeLa cell extract (supplied with kit) was used as a positive control. The assay is developed with a chemiluminescent substrate and the signal is detected using a multimode detector (DTX-880, Beckman Coulter). NF-κB p65 activity was expressed as relative light units (RLUs) and normalized with negative control. Results are representative [mean (
s.e.m
)] of three independent experiments and differ without a common letter; P < 0.05. (c, d) IκBα degradation was analysed by western immunoblotting using antibodies specific for IκBα (Santa Cruz Biotechnology). β-actin was used as protein-loading control. Band images were digitally captured and the band intensities (pixels/band) were obtained using the UN-Scan-It software and are expressed in average pixels. Data shown are cumulative of three experiments and the OD values are mean (
s.e.m
) and differ without a common letter; P < 0.05.
Fig. 8
Activation of NF-κB is required for AGE-BSA-induced expression of IL-6 and IL-8 in primary human OA chondrocytes. Primary human OA chondrocytes were pre-treated with inhibitors specific for IKKα/β (Bay 11-7082), IKKβ (parthenolide), IKKγ (NEMO-BDBP) or proteasome (MG-132) for 1h and then stimulated with AGE-BSA (100 µg/ml) for 24 h. Expression of (a) IL-6 mRNA or (b) IL-8 mRNA was determined as described under Fig. 5. Effect of specific inhibitors of NF-κB on the production of IL-6 (c) and IL-8 (d) in AGE-BSA-stimulated primary human OA chondrocytes. Primary human OA chondrocytes were pre-treated with Bay 11-7082, parthenolide, NEMO-BDBP or MG-132 for 1 h and then stimulated with AGE-BSA (100 µg/ml) for 24 h. Level of IL-6 or IL-8 was quantified by a sandwich ELISA. Primary human OA chondrocytes incubated with native BSA (100 µg/ml) were used as control. Concentration of Bay 11-7082, parthenolide, NEMO-BDBP and MG-132 used was 50, 50, 50 and 100 µM, respectively. Results are representative [mean (
s.e.m
)] of duplicate experiments with OA chondrocytes obtained from age- and sex-matched five OA donors and differ without a common letter P < 0.05.
Similar articles
- Endoplasmic reticulum stress induces the expression of COX-2 through activation of eIF2α, p38-MAPK and NF-κB in advanced glycation end products stimulated human chondrocytes.
Rasheed Z, Haqqi TM. Rasheed Z, et al. Biochim Biophys Acta. 2012 Dec;1823(12):2179-89. doi: 10.1016/j.bbamcr.2012.08.021. Epub 2012 Sep 6. Biochim Biophys Acta. 2012. PMID: 22982228 Free PMC article. - Advanced glycation end-products increase IL-6 and ICAM-1 expression via RAGE, MAPK and NF-κB pathways in human gingival fibroblasts.
Nonaka K, Kajiura Y, Bando M, Sakamoto E, Inagaki Y, Lew JH, Naruishi K, Ikuta T, Yoshida K, Kobayashi T, Yoshie H, Nagata T, Kido J. Nonaka K, et al. J Periodontal Res. 2018 Jun;53(3):334-344. doi: 10.1111/jre.12518. Epub 2017 Nov 30. J Periodontal Res. 2018. PMID: 29193068 - Role of advanced glycation end products in cellular signaling.
Ott C, Jacobs K, Haucke E, Navarrete Santos A, Grune T, Simm A. Ott C, et al. Redox Biol. 2014 Jan 9;2:411-29. doi: 10.1016/j.redox.2013.12.016. eCollection 2014. Redox Biol. 2014. PMID: 24624331 Free PMC article. Review. - Advanced glycation endproducts and osteoarthritis.
Saudek DM, Kay J. Saudek DM, et al. Curr Rheumatol Rep. 2003 Feb;5(1):33-40. doi: 10.1007/s11926-003-0081-x. Curr Rheumatol Rep. 2003. PMID: 12590883 Review.
Cited by
- Impact of diabetes and its treatments on skeletal diseases.
Yan W, Li X. Yan W, et al. Front Med. 2013 Mar;7(1):81-90. doi: 10.1007/s11684-013-0243-9. Epub 2013 Feb 2. Front Med. 2013. PMID: 23377889 Review. - MicroRNA-125b-5p regulates IL-1β induced inflammatory genes via targeting TRAF6-mediated MAPKs and NF-κB signaling in human osteoarthritic chondrocytes.
Rasheed Z, Rasheed N, Abdulmonem WA, Khan MI. Rasheed Z, et al. Sci Rep. 2019 May 3;9(1):6882. doi: 10.1038/s41598-019-42601-3. Sci Rep. 2019. PMID: 31053727 Free PMC article. - Coenzyme Q10 ameliorates pain and cartilage degradation in a rat model of osteoarthritis by regulating nitric oxide and inflammatory cytokines.
Lee J, Hong YS, Jeong JH, Yang EJ, Jhun JY, Park MK, Jung YO, Min JK, Kim HY, Park SH, Cho ML. Lee J, et al. PLoS One. 2013 Jul 22;8(7):e69362. doi: 10.1371/journal.pone.0069362. Print 2013. PLoS One. 2013. PMID: 23894457 Free PMC article. - Serum levels of interleukins and S100A8/A9 correlate with clinical severity in patients with dermatomyositis-associated interstitial lung disease.
Lou Y, Zheng Y, Fan B, Zhang L, Zhu F, Wang X, Chen Z, Tan X, Wei Q. Lou Y, et al. BMC Pulm Med. 2020 Jul 17;20(1):196. doi: 10.1186/s12890-020-01226-3. BMC Pulm Med. 2020. PMID: 32680574 Free PMC article.
References
- Felson DT, Lawrence RC, Dieppe PA, et al. Osteoarthritis: new insights. Part 1: the disease and its risk factors. Ann Intern Med. 2000;133:635–46. - PubMed
- Creamer P, Hochberg MC. Osteoarthritis. Lancet. 1997;350:503–8. - PubMed
- Felson DT, Zhang Y. An update on the epidemiology of knee and hip osteoarthritis with a view to prevention [review] Arthritis Rheum. 1998;41:1343–55. - PubMed
- Hart DJ, Doyle DV, Spector TD. Incidence and risk factors for radiographic knee osteoarthritis in middle-aged women: the Chingford Study. Arthritis Rheum. 1999;42:17–24. - PubMed
- Sell DR, Monnier VM. Structure elucidation of a senescence cross-link from human extracellular matrix: implication of pentoses in the aging process. J Biol Chem. 1989;264:21597–602. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- R01 AT005520/AT/NCCIH NIH HHS/United States
- R21-AT504615/AT/NCCIH NIH HHS/United States
- R01-AT-003267/AT/NCCIH NIH HHS/United States
- R01-AT-005520/AT/NCCIH NIH HHS/United States
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous