Nucleolar disruption in dopaminergic neurons leads to oxidative damage and parkinsonism through repression of mammalian target of rapamycin signaling - PubMed (original) (raw)
Nucleolar disruption in dopaminergic neurons leads to oxidative damage and parkinsonism through repression of mammalian target of rapamycin signaling
Claus Rieker et al. J Neurosci. 2011.
Abstract
The nucleolus represents an essential stress sensor for the cell. However, the molecular consequences of nucleolar damage and their possible link with neurodegenerative diseases remain to be elucidated. Here, we show that nucleolar damage is present in both genders in Parkinson's disease (PD) and in the pharmacological PD model induced by the neurotoxin 1,2,3,6-tetrahydro-1-methyl-4-phenylpyridine hydrochloride (MPTP). Mouse mutants with nucleolar disruption restricted to dopaminergic (DA) neurons show phenotypic alterations that resemble PD, such as progressive and differential loss of DA neurons and locomotor abnormalities. At the molecular level, nucleolar disruption results in increased p53 levels and downregulation of mammalian target of rapamycin (mTOR) activity, leading to mitochondrial dysfunction and increased oxidative stress, similar to PD. In turn, increased oxidative stress induced by MPTP causes mTOR and ribosomal RNA synthesis inhibition. Collectively, these observations suggest that the interplay between nucleolar dysfunction and increased oxidative stress, involving p53 and mTOR signaling, may constitute a destructive axis in experimental and sporadic PD.
Figures
Figure 1.
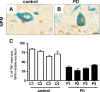
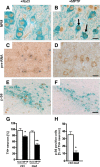
Nucleolar disruption in PD. A, B, Analysis of nucleolar integrity by NPM immunostaining (brown) in DA neurons (TH positive; blue) in age-matched controls and PD patients. C, Quantification of visible nucleoli in four PD samples at different pathology progression show a dramatic decrease of visible nucleoli in TH+ neurons compared with controls. Scale bar: A, B, 20 μm. Error bars represent SEM.
Figure 2.
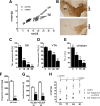
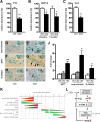
Ablation of TIF-IA in DA neurons leads to parkinsonism in mice. A, Weight curves show growth differences between mutant and control littermates starting at P15 (n = 8). B, Loss of DA neurons in mutant mice at P30, visualized by immunostaining using antibodies against TH. C, D, Quantification of DA neurons at different postnatal stages (P0, P15, P40, and P90), normalized to control littermates and plotted as a histogram for each time point (n = 5), revealed that mutant and control mice did not show any significant differences in number of DA neurons at birth, whereas they are progressively lost at later stages. SN neurons (C) are more susceptible to the loss of TIF-IA than VTA neurons (D). E, Levels of TH immunoreactivity in striata at P7, P15, and P30 were normalized to control littermates and plotted as a histogram for each time point (n = 4). F, The striatal dopamine content measured by HPLC-ED shows a 95% reduction compared with the control group at P40 (n = 5). G, Locomotor deficits of TIF-IADATCre mice determined by the accelerating rotarod assay. At P30, mutants show a locomotor deficit of 55% and at P60 of 76% compared with control mice (n = 5). H, Rescue of TIF-IADATCre mice by
l
-DOPA injection. Mutant mice (>7 weeks of age) treated by daily injecting
l
-DOPA for 3 weeks gained weight similarly to control mice, whereas untreated mutant mice died without gaining weight despite supplementation with liquid food (n = 4). Scale bar, 150 μm. *p < 0.05; **p < 0.01; ***p < 0.001. Error bars represent SEM.
Figure 3.
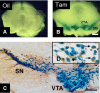
Generation of the inducible DATCreERT2 transgenic mouse line. A, B, Brain sections of the transgenic reporter line ROSA26 expressing the inducible Cre-recombinase injected with either oil or tamoxifen. Whereas in mice injected only with oil, no activity of the reporter gene is detected (A), mice injected with tamoxifen show specific activity of the reporter in the mesencephalic region (B). C, D, The activity of the Cre recombinase is restricted to DA neurons, as determined by X-gal staining (blue) in combination with TH immunohistochemistry (brown). The inset (D) shows a higher magnification of double-stained neurons (arrows). Scale bars: A, B, 150 μm; C, 50 μm.
Figure 4.
Progressive loss of DA neurons in TIF-IADATCreERT2 adult mice. A–F, Loss of TH immunoreactivity in striata is visible in TIF-IADATCreERT2 mutants (B) 7 weeks after tamoxifen injection compared with control (A), whereas TH+ neurons in SN/VTA are minimally affected. Thirteen weeks after injection, TH+ fibers in striata disappear almost completely (D), and severe loss of DA in SN is visible accompanied by partial loss of VTA TH+ neurons. Loss of DA fibers and neurons further advances 21 weeks after induction with tamoxifen (F). No changes are visible in the respective controls at 7, 13, and 21 weeks (A, C, E). G, Quantification of the TH+ immunoreactivity in TIF-IADATCreERT2 mice in the striatum compared with respective control mice (n = 5). H, I, Percentage of remaining DA neurons in SN (H) and VTA (I) of TIF-IADATCreERT2 mutants compared with control littermates (n = 4). J, Measurement of the dopamine content in striata by HPLC-ED reveals in TIF-IADATCreERT2 mice 7 weeks after tamoxifen a ∼45% reduction, reaching ∼80% 30 weeks after tamoxifen (n = 5). K, Locomotor deficits of TIF-IADATCreERT2 mice determined by the accelerating rotarod assay are detected 13 weeks after induction and worsen over time (n = 5). L, Analysis of TH+ neurons in control and TIF-IA; p53DATCreERT2 mice 7 weeks after tamoxifen injection in control (n = 7) and double mutants (DM) (n = 7). Error bars represent SEM. Scale bars: A–F, top panels, 400 μm; bottom panels, 150 μm. *p < 0.05; **p < 0.01; ***p < 0.001.
Figure 5.
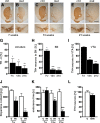
Oxidative stress after MPTP treatment affects nucleolar function. A, B, Analysis of nucleolar integrity in DA neurons by immunohistochemistry using antibodies against NPM (brown) and TH (green) in 2-month-old wild-type mice injected for 3 d with either MPTP or NaCl and analyzed 1 d after the last injection (n = 5). C, D, Effect on 47S pre-rRNA synthesis in DA neurons after NaCl (C) or MPTP injection (D) analyzed by in situ hybridization with a riboprobe for the 5′-ETS of the 47S pre-rRNA (blue) in combination with TH immunohistochemistry (brown) to identify DA neurons. E, F, IHC using antibodies against p-S6 (brown) and TH (light blue) shows a decrease of phosho-S6 (p-S6) in MPTP-treated wild-type mice. G, Effect of treatment with the neurotoxin MPTP on the number of TH+ neurons in control and TIF-IADATCreERT2 mutant mice 2 weeks after tamoxifen (n = 5). Control mice show moderate loss of DA neurons on MPTP treatment, whereas TIF-IADATCreERT2 mutants treated with the neurotoxin show a more severe reduction of TH+ neurons. H, Quantification of the number of p-S6-positive DA neurons in TIF-IADATCreERT2 and control littermates 4 weeks after tamoxifen. Scale bars: A, B, 20 μm; C, D, 25 μm; E, F, 50 μm. *p < 0.05; **p < 0.01. Error bars represent SEM.
Figure 6.
Perturbation of rRNA synthesis leads to mitochondrial impairment followed by increased oxidative stress. A, The bars represent abundance of YY1 transcripts by qPCR normalized to HPRT in control and TIF-IADATCreERT2 mice 2 weeks after tamoxifen (n = 3). B, The graph shows UCP-2 gene expression by qPCR. The bars represent abundance of UCP-2 transcripts normalized to the levels HPRT 2 and 3 weeks after tamoxifen treatment (n = 3). C, Two weeks after tamoxifen injection, reduced COX activity in TIF-IADATCreERT2 mutants is measured as optical density compared with control mice (n = 3). D–I, Brain sections through the ventral midbrain were analyzed for neuroketals (D, E) as marker for ROS-induced lipid damage (brown), for nitrosylated proteins (NITT) (F, G), for 8-hydroxydeoxyguanosine (8-OHdG) (H, I), as marker for ROS-induced DNA damage (brown), in combination with TH staining (light blue) to identify DA. The insets show higher magnification of immunostained cells. Scale bars, 50 μm. J, Quantification of DA neurons positive for oxidative stress markers NITT, neuroketals, and 8-OHdG in control and mutant mice 2 and 4 weeks after injection with tamoxifen. Differences are expressed as fold change compared with the mean of the controls at different stages. Higher levels of oxidative stress markers were observed in the mutants (n = 4). *p < 0.05; **p < 0.01. Error bars represent SEM. K, Diagram showing the sequence of events triggered by nucleolar damage. The decrease and increase of the parameters indicated (left) are depicted in green or red, respectively. The bars indicate the time points (weeks) analyzed after injection of tamoxifen to induce TIF-IA loss in adult mice. L, Schematic representation showing the molecular mechanisms shared between TIF-IA mutation and MPTP pharmacological models. Nucleolar damage as a consequence of TIF-IA mutation induces p53 and inhibits mTOR, causing mitochondrial dysfunction and increased oxidative damage. In the pharmacological model, mitochondrial dysfunction caused by MPTP leads to increased oxidative stress. This increase inhibits mTOR and rRNA synthesis, probably by downregulation of TIF-IA activity, causing nucleolar damage and additional consequences on cell survival.
Similar articles
- Pten ablation in adult dopaminergic neurons is neuroprotective in Parkinson's disease models.
Domanskyi A, Geissler C, Vinnikov IA, Alter H, Schober A, Vogt MA, Gass P, Parlato R, Schütz G. Domanskyi A, et al. FASEB J. 2011 Sep;25(9):2898-910. doi: 10.1096/fj.11-181958. Epub 2011 May 18. FASEB J. 2011. PMID: 21593433 - How Parkinson's disease meets nucleolar stress.
Parlato R, Liss B. Parlato R, et al. Biochim Biophys Acta. 2014 Jun;1842(6):791-7. doi: 10.1016/j.bbadis.2013.12.014. Epub 2014 Jan 8. Biochim Biophys Acta. 2014. PMID: 24412806 Review. - Alkaloids extracted from Uncaria rhynchophylla demonstrate neuroprotective effects in MPTP-induced experimental parkinsonism by regulating the PI3K/Akt/mTOR signaling pathway.
Zheng M, Chen M, Liu C, Fan Y, Shi D. Zheng M, et al. J Ethnopharmacol. 2021 Feb 10;266:113451. doi: 10.1016/j.jep.2020.113451. Epub 2020 Oct 10. J Ethnopharmacol. 2021. PMID: 33049346 - p53 inhibitors preserve dopamine neurons and motor function in experimental parkinsonism.
Duan W, Zhu X, Ladenheim B, Yu QS, Guo Z, Oyler J, Cutler RG, Cadet JL, Greig NH, Mattson MP. Duan W, et al. Ann Neurol. 2002 Nov;52(5):597-606. doi: 10.1002/ana.10350. Ann Neurol. 2002. PMID: 12402257 - [Parkinson's disease and nucleolar stress].
Zhou Q, Chen Y, Wei Q, Shang H. Zhou Q, et al. Zhonghua Yi Xue Yi Chuan Xue Za Zhi. 2016 Jun;33(3):392-5. doi: 10.3760/cma.j.issn.1003-9406.2016.03.026. Zhonghua Yi Xue Yi Chuan Xue Za Zhi. 2016. PMID: 27264829 Review. Chinese.
Cited by
- Insights into the regulation of neuronal viability by nucleophosmin/B23.
Pfister JA, D'Mello SR. Pfister JA, et al. Exp Biol Med (Maywood). 2015 Jun;240(6):774-86. doi: 10.1177/1535370215579168. Epub 2015 Apr 22. Exp Biol Med (Maywood). 2015. PMID: 25908633 Free PMC article. Review. - Altered nucleolar morphology in substantia nigra dopamine neurons following 6-hydroxydopamine lesion in rats.
Healy-Stoffel M, Ahmad SO, Stanford JA, Levant B. Healy-Stoffel M, et al. Neurosci Lett. 2013 Jun 24;546:26-30. doi: 10.1016/j.neulet.2013.04.033. Epub 2013 May 2. Neurosci Lett. 2013. PMID: 23643997 Free PMC article. - Shared GABA transmission pathology in dopamine agonist- and antagonist-induced dyskinesia.
Abe Y, Yagishita S, Sano H, Sugiura Y, Dantsuji M, Suzuki T, Mochizuki A, Yoshimaru D, Hata J, Matsumoto M, Taira S, Takeuchi H, Okano H, Ohno N, Suematsu M, Inoue T, Nambu A, Watanabe M, Tanaka KF. Abe Y, et al. Cell Rep Med. 2023 Oct 17;4(10):101208. doi: 10.1016/j.xcrm.2023.101208. Epub 2023 Sep 28. Cell Rep Med. 2023. PMID: 37774703 Free PMC article. - Epigenetic silencing of nucleolar rRNA genes in Alzheimer's disease.
Pietrzak M, Rempala G, Nelson PT, Zheng JJ, Hetman M. Pietrzak M, et al. PLoS One. 2011;6(7):e22585. doi: 10.1371/journal.pone.0022585. Epub 2011 Jul 22. PLoS One. 2011. PMID: 21799908 Free PMC article. - Age and α-synuclein expression interact to reveal a dependence of dopaminergic axons on endogenous Akt/PKB signaling.
Kim SR, Ries V, Cheng HC, Kareva T, Oo TF, Yu WH, Duff K, Kholodilov N, Burke RE. Kim SR, et al. Neurobiol Dis. 2011 Nov;44(2):215-22. doi: 10.1016/j.nbd.2011.07.003. Epub 2011 Jul 18. Neurobiol Dis. 2011. PMID: 21782946 Free PMC article.
References
- Abou-Sleiman PM, Muqit MM, Wood NW. Expanding insights of mitochondrial dysfunction in Parkinson's disease. Nat Rev Neurosci. 2006;7:207–219. - PubMed
- Bae BI, Xu H, Igarashi S, Fujimuro M, Agrawal N, Taya Y, Hayward SD, Moran TH, Montell C, Ross CA, Snyder SH, Sawa A. p53 mediates cellular dysfunction and behavioral abnormalities in Huntington's disease. Neuron. 2005;47:29–41. - PubMed
- Boisvert FM, van Koningsbruggen S, Navascués J, Lamond AI. The multifunctional nucleolus. Nat Rev Mol Cell Biol. 2007;8:574–585. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials
Miscellaneous