Myeloperoxidase-specific plasma cell depletion by bortezomib protects from anti-neutrophil cytoplasmic autoantibodies-induced glomerulonephritis - PubMed (original) (raw)
Myeloperoxidase-specific plasma cell depletion by bortezomib protects from anti-neutrophil cytoplasmic autoantibodies-induced glomerulonephritis
Julia Bontscho et al. J Am Soc Nephrol. 2011 Feb.
Abstract
Anti-neutrophil cytoplasmic autoantibodies (ANCA) cause vasculitis and necrotizing crescentic glomerulonephritis (NCGN). Steroids and cytotoxic drugs reduce mortality but can cause significant adverse events. The proteasome inhibitor bortezomib (BTZ) prevents glomerulonephritis in mouse models of lupus but its efficacy in ANCA-associated glomerulonephritis is unknown. We induced anti-MPO IgG-mediated NCGN by transplanting wild-type bone marrow (BM) into irradiated MPO-deficient mice immunized with MPO. Four weeks after BM transplantation, we treated mice with steroid/cyclophosphamide (S/CYC) or BTZ. Compared with untreated control mice, both S/CYC and BTZ significantly reduced urine abnormalities, NCGN, and infiltration of neutrophils and macrophages. Response to BTZ depended on timing of administration: BTZ abrogated NCGN if begun 3 weeks, but not 5 weeks, after BM transplantation. BTZ treatment significantly reduced total and MPO-specific plasma cells in both the spleen and bone marrow, resulting in significantly reduced anti-MPO titers. Furthermore, BTZ affected neither B cells nor total CD4 and CD8 T cells, including their naive and effector subsets. In contrast, S/CYC reduced the total number of cells in the spleen, including total and MPO-specific plasma cells and B cells. In contrast to BTZ, S/CYC did not affect total and MPO-specific plasma cells in the bone marrow. Three of 23 BTZ-treated mice died within 36 hours after BTZ administration. In summary, BTZ depletes MPO-specific plasma cells, reduces anti-MPO titers, and prevents NCGN in mice.
Figures
Figure 1.
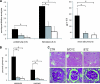
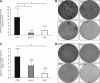
BTZ and S/CYC treatment prevents ANCA-induced necrotizing crescentic glomerulonephritis. Urine and renal histology in controls (black columns, CTR) or in mice treated with S/CYC (gray) and BTZ (white), respectively. Mice were sacrificed after 4 weeks of treatment. Panel (A) shows dipstick analysis and albuminuria ELISA. Panel (B) depicts renal tissue analysis; glomerular crescents and necrosis were expressed as the mean percentage of glomeruli with crescents and necrosis. Typical examples for each treatment group are depicted with ×20 magnification in the upper row and ×40 magnification in the lower row in panel (C). *P < 0.05.
Figure 2.
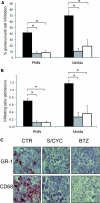
BTZ and S/CYC treatments diminish glomerular PMN and macrophage influx. Panel (A) shows the percentage of glomeruli with PMN or macrophage infiltration and panel (B) the absolute number of these cells per glomerulus. Representative cross sections with GR-1 staining for PMN and CD68 staining for macrophages are depicted in panel (C). The groups consist of controls (CTR, black columns), S/CYC-treated mice (gray), and BTZ-treated mice (white). *P < 0.05.
Figure 3.
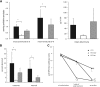
BTZ reduces anti-MPO antibody titer. Results are shown in arbitrary units (405) nm. The anti-MPO titer was measured at randomization, after 1 week of treatment, and at sacrifice after 3 to 4 weeks of treatment. * indicates a significant difference compared with the control group at the indicated time point. # indicates a significant difference compared with the titer at randomization. P < 0.05.
Figure 4.
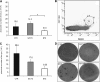
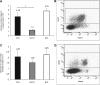
BTZ does not reduce the number of total splenic plasma cells. Panel (A) shows the effect of S/CYC (gray columns) and BTZ (white) in comparison to untreated control mice (CTR, black) on the absolute number of splenic plasma cells analyzed by flow cytometry. Panel (B) depicts a typical experiment in a control mouse. The gated plasma cells express CD138 and cytoplasmic Ig κ light chains (cIg-κ). Panel (C) gives the absolute number of IgG and IgM secreting splenic plasma cells by ELISPOT with a typical example in panel (D). *P < 0.05.
Figure 5.
BTZ and S/CYC do not reduce the number of BM plasma cells. Panel (A) shows the effect of S/CYC (gray columns) and BTZ (white) in comparison to control mice (CTR, black) on the absolute number of BM plasma cells analyzed by flow cytometry. Panel (B) depicts a typical experiment in a control mouse. The gated plasma cells express CD138 and cytoplasmic Ig κ light chains (cIg-κ). Panel (C) gives the absolute number of IgG and IgM secreting BM plasma cells by ELISPOT with a typical example in panel (D). *P < 0.05.
Figure 6.
BTZ reduces MPO-specific plasma cells in spleen and BM. Panel (A) shows the effect of S/CYC (gray columns) and BTZ (white) in comparison to control mice (CTR, black) on the absolute number of MPO-specific splenic plasma cells. Panel (C) shows MPO-specific BM plasma cells. Panel (B) (splenic) and panel (D) (BM) depict typical experiments. No SAP means that the substrate streptavidin-AP was omitted. *P < 0.05.
Figure 7.
BTZ does not affect the number of B cells in spleen and bone marrow. Panel (A) shows the effect of S/CYC (gray columns) and BTZ (white) in comparison to control mice (CTR, black) on the absolute number of splenic B220-positive B lymphocytes analyzed by flow cytometry. Panel (B) depicts a typical experiment in a control mouse. Panel (C) shows the effects on the absolute number of BM B lymphocytes with a typical example in panel (D). *P < 0.05.
Figure 8.
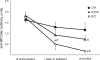
Early but not late BTZ treatment protects from ANCA-induced NCGN. Urine dipstick analysis and albuminuria were measured at sacrifice (A) and renal tissue was examined (B). Black columns depict controls (CTR), white columns early BTZ, and dark gray columns late BTZ treatment. Glomerular crescents and necrosis were expressed as the mean percentage of glomeruli with crescents and necrosis. The treatment effect on circulating anti-MPO titers was assessed by ELISA. Results are shown in arbitrary units at outside diameter 405 nm. The anti-MPO titer was measured at random, 1 week after early BTZ treatment had started, and at sacrifice. * indicates a significant difference compared with the control group at the indicated time point. # indicates a significant difference compared with the titer at randomization. P < 0.05.
Figure 9.
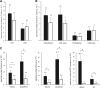
Early BTZ treatment has no effect on splenic T cells and reduces total and MPO-specific plasma cells. Panel (A) shows the effect of early BTZ treatment on the absolute number of splenic CD4 and CD8 T cells, panel (B) the effect on the CD4 and CD8 effector and naive subtypes, and panel (C) the effect of early BTZ treatment on the absolute number of splenic and BM total and MPO-specific plasma cells analyzed by flow cytometry and ELISPOT. White columns depicts early BTZ treatment and black columns the control group. *P < 0.05.
Similar articles
- Bone marrow-derived cells are sufficient and necessary targets to mediate glomerulonephritis and vasculitis induced by anti-myeloperoxidase antibodies.
Schreiber A, Xiao H, Falk RJ, Jennette JC. Schreiber A, et al. J Am Soc Nephrol. 2006 Dec;17(12):3355-64. doi: 10.1681/ASN.2006070718. Epub 2006 Nov 15. J Am Soc Nephrol. 2006. PMID: 17108314 - Neutrophil Gelatinase-Associated Lipocalin Protects from ANCA-Induced GN by Inhibiting TH17 Immunity.
Schreiber A, Rousselle A, Klocke J, Bachmann S, Popovic S, Bontscho J, Schmidt-Ott KM, Siffrin V, Jerke U, Ashraf MI, Panzer U, Kettritz R. Schreiber A, et al. J Am Soc Nephrol. 2020 Jul;31(7):1569-1584. doi: 10.1681/ASN.2019090879. Epub 2020 Jun 2. J Am Soc Nephrol. 2020. PMID: 32487561 Free PMC article. - IgG glycan hydrolysis attenuates ANCA-mediated glomerulonephritis.
van Timmeren MM, van der Veen BS, Stegeman CA, Petersen AH, Hellmark T, Collin M, Heeringa P. van Timmeren MM, et al. J Am Soc Nephrol. 2010 Jul;21(7):1103-14. doi: 10.1681/ASN.2009090984. Epub 2010 May 6. J Am Soc Nephrol. 2010. PMID: 20448018 Free PMC article. - Neutrophil functions of patients with vasculitis related to myeloperoxidase-specific anti-neutrophil antibody.
Suzuki K. Suzuki K. Int J Hematol. 2001 Aug;74(2):134-43. doi: 10.1007/BF02981995. Int J Hematol. 2001. PMID: 11594512 Review. - Antiproteinase 3- and antimyeloperoxidase-associated vasculitis.
Franssen CF, Stegeman CA, Kallenberg CG, Gans RO, De Jong PE, Hoorntje SJ, Tervaert JW. Franssen CF, et al. Kidney Int. 2000 Jun;57(6):2195-206. doi: 10.1046/j.1523-1755.2000.00080.x. Kidney Int. 2000. PMID: 10844589 Review.
Cited by
- Cold shock Y-box protein-1 proteolysis autoregulates its transcriptional activities.
van Roeyen CR, Scurt FG, Brandt S, Kuhl VA, Martinkus S, Djudjaj S, Raffetseder U, Royer HD, Stefanidis I, Dunn SE, Dooley S, Weng H, Fischer T, Lindquist JA, Mertens PR. van Roeyen CR, et al. Cell Commun Signal. 2013 Aug 27;11:63. doi: 10.1186/1478-811X-11-63. Cell Commun Signal. 2013. PMID: 24103640 Free PMC article. - Impact of intravenous immunoglobulin on the dopaminergic system and immune response in the acute MPTP mouse model of Parkinson's disease.
St-Amour I, Bousquet M, Paré I, Drouin-Ouellet J, Cicchetti F, Bazin R, Calon F. St-Amour I, et al. J Neuroinflammation. 2012 Oct 9;9:234. doi: 10.1186/1742-2094-9-234. J Neuroinflammation. 2012. PMID: 23046563 Free PMC article. - High-affinity autoreactive plasma cells disseminate through multiple organs in patients with immune thrombocytopenic purpura.
Canales-Herrerias P, Crickx E, Broketa M, Sokal A, Chenon G, Azzaoui I, Vandenberghe A, Perima A, Iannascoli B, Richard-Le Goff O, Castrillon C, Mottet G, Sterlin D, Robbins A, Michel M, England P, Millot GA, Eyer K, Baudry J, Mahevas M, Bruhns P. Canales-Herrerias P, et al. J Clin Invest. 2022 Jun 15;132(12):e153580. doi: 10.1172/JCI153580. J Clin Invest. 2022. PMID: 35503254 Free PMC article. - Targeting B Cells and Plasma Cells in Glomerular Diseases: Translational Perspectives.
Schrezenmeier E, Jayne D, Dörner T. Schrezenmeier E, et al. J Am Soc Nephrol. 2018 Mar;29(3):741-758. doi: 10.1681/ASN.2017040367. Epub 2018 Jan 11. J Am Soc Nephrol. 2018. PMID: 29326157 Free PMC article. Review. - Alterations in the ubiquitin proteasome system in persistent but not reversible proteinuric diseases.
Beeken M, Lindenmeyer MT, Blattner SM, Radón V, Oh J, Meyer TN, Hildebrand D, Schlüter H, Reinicke AT, Knop JH, Vivekanandan-Giri A, Münster S, Sachs M, Wiech T, Pennathur S, Cohen CD, Kretzler M, Stahl RA, Meyer-Schwesinger C. Beeken M, et al. J Am Soc Nephrol. 2014 Nov;25(11):2511-25. doi: 10.1681/ASN.2013050522. Epub 2014 Apr 10. J Am Soc Nephrol. 2014. PMID: 24722446 Free PMC article.
References
- van der Woude FJ, Rasmussen N, Lobatto S, Wiik A, Permin H, van Es LA, van der Giessen M, van der Hem GK, The TH: Autoantibodies against neutrophils and monocytes: Tool for diagnosis and marker for disease activity in Wegener's granulomatosis. Lancet 1(8426): 425–429, 1985 - PubMed
- Falk RJ, Jennette JC: Anti-neutrophil cytoplasmic autoantibodies with specificity for myeloperoxidase in patients with systemic vasculitis and idiopathic necrotizing and crescentic glomerulonephritis. N Engl J Med 318: 1651–1657, 1988 - PubMed
- Kain R, Matsui K, Exner M, Binder S, Schaffner G, Sommer EM, Kerjaschki D: A novel class of autoantigens of anti-neutrophil cytoplasmic antibodies in necrotizing and crescentic glomerulonephritis: The lysosomal membrane glycoprotein h-lamp-2 in neutrophil granulocytes and a related membrane protein in glomerular endothelial cells. J Exp Med 181: 585–597, 1995 - PMC - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous