Beta interferon suppresses the development of experimental cerebral malaria - PubMed (original) (raw)
Beta interferon suppresses the development of experimental cerebral malaria
Craig N Morrell et al. Infect Immun. 2011 Apr.
Abstract
Cerebral malaria (CM) is a major complication of Plasmodium falciparum infection, particularly in children. The pathogenesis of cerebral malaria involves parasitized red blood cell (RBC)-mediated vascular inflammation, immune stimulation, loss of blood-brain barrier integrity, and obstruction of cerebral capillaries. Therefore, blunting vascular inflammation and immune cell recruitment is crucial in limiting the disease course. Beta interferon (IFN-β) has been used in the treatment of diseases, such as multiple sclerosis (MS) but has not yet been explored in the treatment of CM. Therefore, we sought to determine whether IFN-β also limits disease progression in experimental cerebral malaria (ECM). Plasmodium berghei-infected mice treated with IFN-β died later and showed increased survival, with improved blood-brain barrier function, compared to infected mice. IFN-β did not alter systemic parasitemia. However, we identified multiple action sites that were modified by IFN-β administration. P. berghei infection resulted in increased expression of chemokine (C-X-C motif) ligand 9 (CXCL9) in brain vascular endothelial cells that attract T cells to the brain, as well as increased T-cell chemokine (C-X-C motif) receptor 3 (CXCR3) expression. The infection also increased the cellular content of intercellular adhesion molecule 1 (ICAM-1), a molecule important for attachment of parasitized RBCs to the endothelial cell. In this article, we report that IFN-β treatment leads to reduction of CXCL9 and ICAM-1 in the brain, reduction of T-cell CXCR3 expression, and downregulation of serum tumor necrosis factor alpha (TNF-α). In addition, IFN-β-treated P. berghei-infected mice also had fewer brain T-cell infiltrates, further demonstrating its protective effects. Hence, IFN-β has important anti-inflammatory properties that ameliorate the severity of ECM and prolong mouse survival.
Figures
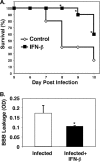
FIG. 1.
IFN-β is protective in experimental cerebral malaria (ECM). (A) Survival. Mice were infected with Plasmodium berghei, and their survival was monitored. The values that were significantly different (P < 0.05) from the value for control, non-IFN-β-treated mice are indicated by an asterisk. (B) Blood-brain barrier (BBB). On day 5 postinfection, the mice were injected with Evans blue dye, and dye extravasation was measured (n = 5). Values are means plus standard errors of the means (SEMs) (error bars). The value for infected and IFN-β-treated mice was significantly different (P < 0.05) from the value for infected mice and is indicated by an asterisk. OD, optical density.
FIG. 2.
Brain pathology in the cerebral cortex. On day 5 postinfection, brains were collected from mice, and hematoxylin and eosin staining was performed. The arrows point to areas of vascular inflammation.
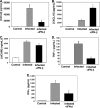
FIG. 3.
IFN-β suppresses ECM-associated inflammation. On day 5 postinfection, mouse plasma was isolated and evaluated by EIA. (A to E) The concentrations of CXCL9 (A), CXCL10 (B), sICAM-1 (C), TNF-α (D), and IFN-γ (E) in the three groups of mice are shown. (A) CXCL9 (n = 5). Values are means plus standard deviation (SDs) (error bars). The value that was significantly different (P < 0.05) from the value for infected mice is indicated by an asterisk. (B) CXCL10 (n = 4 or 5). The value that was significantly different (P < 0.01) from the value for infected mice is indicated by an asterisk. (C) sICAM-1 (n = 5). (D) TNF-α (n = 3). The value that was significantly different (P < 0.01) from the value for infected mice is indicated by an asterisk. (E) IFN-γ (n = 3). The value that was significantly different (P = 0.02) from the value for infected mice is indicated by an asterisk. Values in panels B, D, and E are means plus SEMs (error bars).
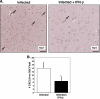
FIG. 4.
IFN-β alters brain CXCL9 production. (A) CXCL9 immunohistochemical analysis. The arrows point to blood vessels that are positive for CXCL9. (B) Quantification of CXCL9-positive vessels. Infected mice had approximately 14 CXCL9-positive (+ve) vessels per 20× field, whereas in IFN-β-treated mice, the number of such vessels was reduced to approximately 7 (5 fields per mouse; 5 mice). Values are means plus SDs (error bars). The value for infected and IFN-β-treated mice was significantly different (P < 0.01) from the value for infected mice and is indicated by an asterisk.
FIG. 5.
IFN-β alters brain CXCL10 production. (A) CXCL10 immunohistochemical analysis. The arrows point to CXCL10-positive astrocytes and glial cells. (B) Quantification of CXCL10-positive cells. Infected mice had approximately 40 CXCL10-positive cells per 20× field, whereas in IFN-β-treated mice, this number was increased to greater than 80 (5 fields per mouse; five mice). Values are means plus SDs (error bars). The value for infected and IFN-β-treated mice was significantly different (P < 0.01) from the value for infected mice and is indicated by an asterisk.
FIG. 6.
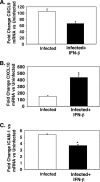
IFN-β alters expression of CXCL9, CXCL10, and ICAM-1 mRNA in the brain. (A) CXCL9 (n = 3 or 4). (B) CXCL10 (n = 3 or 4). The value for infected and IFN-β-treated mice was significantly different (P < 0.05) from the value for infected mice and is indicated by an asterisk. (C) ICAM-1 (n = 3 or 4). The value for infected and IFN-β-treated mice was significantly different (P < 0.02) from the value for infected mice and is indicated by an asterisk. Values are means plus SEMs (error bars). The mRNA levels for each primer pair were normalized to the housekeeping gene GAPDH rRNA level, and fold changes were calculated against control uninfected mice.
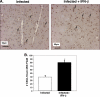
FIG. 7.
IFN-β reduces T-cell trafficking in ECM. T-cell trafficking to the brain in ECM was evaluated by immunohistochemical staining with anti-CD3 antibody.
FIG. 8.
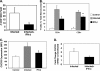
T-cell trafficking in ECM is reduced by IFN-β. (A) Quantification of CD3-positive cells taken from Fig. 7. Infected mice had approximately 25 CD3-positive cells per 20× field, but the number was reduced to less than 10 in IFN-β-treated mice. There were 5 fields per mouse and 5 mice. The value for infected and IFN-β-treated mice was significantly different (P < 0.01) from the value for infected mice and is indicated by an asterisk. In panels A to C, values are means plus SDs (error bars). (B) Isolated T-cell subsets from mouse brains. Mononuclear cells were isolated from the brains of control mice, infected mice, and infected mice treated with IFN-β. T cells were quantified by FACS (n = 5). The value that was significantly different (P < 0.06) from the value for infected, CD8-positive cells is indicated by an asterisk. The value that was significantly different (P < 0.05) from the value for infected, CD4-positive cells is indicated by two asterisks. (C) T-cell CXCR3 expression. Splenocytes were isolated from control, infected, and infected mice treated with IFN-β. T-cell CXCR3 expression was determined by FACS (n = 5). The value that was significantly different (P < 0.05) from the value for infected mice is indicated by an asterisk. MFI, mean fluorescence intensity. (D) CXCR3 mRNA expression in the brain by qRT-PCR (n = 3 or 4). Values are means plus SEMs (error bars). The mRNA levels for each primer pair were normalized to the housekeeping gene GAPDH rRNA level, and fold changes were calculated against control uninfected mice.
FIG. 9.
Diagram of the hypothesized actions of IFN-β on the pathogenesis of cerebral malaria.
Similar articles
- Type I interferons contribute to experimental cerebral malaria development in response to sporozoite or blood-stage Plasmodium berghei ANKA.
Palomo J, Fauconnier M, Coquard L, Gilles M, Meme S, Szeremeta F, Fick L, Franetich JF, Jacobs M, Togbe D, Beloeil JC, Mazier D, Ryffel B, Quesniaux VF. Palomo J, et al. Eur J Immunol. 2013 Oct;43(10):2683-95. doi: 10.1002/eji.201343327. Epub 2013 Jul 19. Eur J Immunol. 2013. PMID: 23780878 - Chemokine receptor CXCR3 and its ligands CXCL9 and CXCL10 are required for the development of murine cerebral malaria.
Campanella GS, Tager AM, El Khoury JK, Thomas SY, Abrazinski TA, Manice LA, Colvin RA, Luster AD. Campanella GS, et al. Proc Natl Acad Sci U S A. 2008 Mar 25;105(12):4814-9. doi: 10.1073/pnas.0801544105. Epub 2008 Mar 17. Proc Natl Acad Sci U S A. 2008. PMID: 18347328 Free PMC article. - Role of ICAM-1 (CD54) in the development of murine cerebral malaria.
Favre N, Da Laperousaz C, Ryffel B, Weiss NA, Imhof BA, Rudin W, Lucas R, Piguet PF. Favre N, et al. Microbes Infect. 1999 Oct;1(12):961-8. doi: 10.1016/s1286-4579(99)80513-9. Microbes Infect. 1999. PMID: 10617927 - Tumor necrosis factor alpha in the pathogenesis of cerebral malaria.
Gimenez F, Barraud de Lagerie S, Fernandez C, Pino P, Mazier D. Gimenez F, et al. Cell Mol Life Sci. 2003 Aug;60(8):1623-35. doi: 10.1007/s00018-003-2347-x. Cell Mol Life Sci. 2003. PMID: 14504653 Free PMC article. Review. - Pathogenesis of cerebral malaria: recent experimental data and possible applications for humans.
Lou J, Lucas R, Grau GE. Lou J, et al. Clin Microbiol Rev. 2001 Oct;14(4):810-20, table of contents. doi: 10.1128/CMR.14.4.810-820.2001. Clin Microbiol Rev. 2001. PMID: 11585786 Free PMC article. Review.
Cited by
- Host immune response in returning travellers infected with malaria.
MacMullin G, Mackenzie R, Lau R, Khang J, Zhang H, Rajwans N, Liles WC, Pillai DR. MacMullin G, et al. Malar J. 2012 May 3;11:148. doi: 10.1186/1475-2875-11-148. Malar J. 2012. PMID: 22554058 Free PMC article. - Efficacy and safety evaluation of a novel trioxaquine in the management of cerebral malaria in a mouse model.
Odhiambo OC, Wamakima HN, Magoma GN, Kirira PG, Malala BJ, Kimani FT, Muregi FW. Odhiambo OC, et al. Malar J. 2017 Jul 3;16(1):268. doi: 10.1186/s12936-017-1917-6. Malar J. 2017. PMID: 28673299 Free PMC article. - The blood transcriptome of childhood malaria.
Boldt ABW, van Tong H, Grobusch MP, Kalmbach Y, Dzeing Ella A, Kombila M, Meyer CG, Kun JFJ, Kremsner PG, Velavan TP. Boldt ABW, et al. EBioMedicine. 2019 Feb;40:614-625. doi: 10.1016/j.ebiom.2018.12.055. Epub 2019 Jan 10. EBioMedicine. 2019. PMID: 30638864 Free PMC article. - Interferons and interferon regulatory factors in malaria.
Gun SY, Claser C, Tan KS, Rénia L. Gun SY, et al. Mediators Inflamm. 2014;2014:243713. doi: 10.1155/2014/243713. Epub 2014 Jul 15. Mediators Inflamm. 2014. PMID: 25157202 Free PMC article. Review. - Cerebral malaria: gamma-interferon redux.
Hunt NH, Ball HJ, Hansen AM, Khaw LT, Guo J, Bakmiwewa S, Mitchell AJ, Combes V, Grau GE. Hunt NH, et al. Front Cell Infect Microbiol. 2014 Aug 15;4:113. doi: 10.3389/fcimb.2014.00113. eCollection 2014. Front Cell Infect Microbiol. 2014. PMID: 25177551 Free PMC article. Review.
References
- Chakravorty, S. J., and A. Craig. 2005. The role of ICAM-1 in Plasmodium falciparum cytoadherence. Eur. J. Cell Biol. 84:15-27. - PubMed
- Frangogiannis, N. G. 2007. Chemokines in ischemia and reperfusion. Thromb. Haemost. 97:738-747. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous