Total synthesis and biological evaluation of a series of macrocyclic hybrids and analogues of the antimitotic natural products dictyostatin, discodermolide, and taxol - PubMed (original) (raw)
Total synthesis and biological evaluation of a series of macrocyclic hybrids and analogues of the antimitotic natural products dictyostatin, discodermolide, and taxol
Ian Paterson et al. Chem Asian J. 2011.
Abstract
The design, synthesis, and biological evaluation of a series of hybrids and analogues of the microtubule-stabilizing anticancer agents dictyostatin, discodermolide, and taxol is described. A 22-membered macrolide scaffold was prepared by adapting earlier synthetic routes directed towards dictyostatin and discodermolide, taking advantage of the distinctive structural and stereochemical similarities between these two polyketide-derived marine natural products. Initial endeavors towards accessing novel discodermolide/dictyostatin hybrids led to the adoption of a late-stage diversification strategy and the construction of a small library of methyl-ether derivatives, along with the first triple hybrids bearing the side-chain of taxol or taxotere attached through an ester linkage. Biological assays of the anti-proliferative activity of these compounds in a series of human cancer cell lines, including the taxol-resistant NCI/ADR-Res cell line, allowed the proposal of various structure-activity relationships. This led to the identification of a potent macrocyclic discodermolide/dictyostatin hybrid 12 and its C9 methoxy derivative 38, accessible by an efficient total synthesis and with a similar biological profile to dictyostatin.
Copyright © 2011 WILEY-VCH Verlag GmbH & Co. KGaA, Weinheim.
Figures
Figure 1
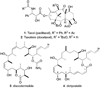
Microtubule-stabilising agents Taxol (paclitaxel, 1), Taxotere (docetaxel, 2), discodermolide (3) and dictyostatin (4).
Figure 2
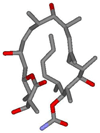
The lowest energy conformer of discodermolide in D2O as deduced by Canales et al This hairpin conformation is broadly similar to the single-crystal X-ray structure of discodermolide in the solid state.
Figure 3
The microtubule-bound bioactive conformations of discodermolide (green) and dictyostatin (blue) overlaid at the taxoid binding site on β-tubulin, as calculated with AutoDock by Canales et al (Image 1). In Image 2, taxol (red) has also been included, the additional region of the binding pocket exploited by the C13 ester side chain can be distinguished.
Figure 4
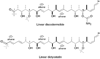
Representations of C1–C24 and C1–C26 carbon chains of discodermolide (3) and dictyostatin (4) drawn in a linear manner to allow comparison of the matching stereochemistry and structural features.
Figure 5
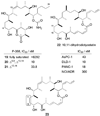
Reduced derivatives of discodermolide with various levels of saturation (19, 20 and 21) and structures of 10,11-dihydro analogues 22 and 23 of dictyostatin and hybrid 12 respectively.
Figure 6
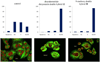
(Graphs) Cell cycle analysis by flow cytometry of PANC-1 cells incubated for 24 h with DMSO (control), 100 nM dictyostatin/discodermolide hybrid 12 or 9-methoxy derivative 38. Histograms represent samples of approximately 1 × 104 cells per test and are plotted as percentage (_y_-axis) vs stage of cell cycle (_x_-axis). Both compounds result in an accumulation of cells in the G2/M phase. (Images) Immunofluorescence images of PANC-1 cells stained with anti-α-tubulin (green) and propidium iodide (red) and observed with confocal microscopy. Cells were exposed to DMSO (control), 100 nM 12 or 100 nM 38. Dense microtubule bundling can be seen around the nuclei on treatment with 12 and 38, a characteristic feature of microtubule-stabilising agents.
Scheme 1
Retrosynthetic analysis of dictyostatin/discodermolide hybrid 5.
Scheme 2
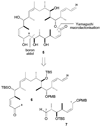
Generation of aldol adduct 10. a) BCl3·DMS, CH2Cl2, −78 → 0 °C, 2 h; b) TEMPO, PhI(OAc)2, CH2Cl2, 20 °C, 2 h; c) K2CO3, 18-crown-6, methyl-_P, P, bis_-(2,2,2-trifluoroethyl)phosphonoacetate, PhMe / HMPA, 0 °C, 16 h; d) 1. 6, Et3N, _c_-Hex2BCl, Et2O, 0 °C, 1 h; 7, −78 °C, 15 min; 2. pH 7 buffer (>95 : 5 dr, 48%). DMS = dimethyl sulfide; HMPA = hexamethylphosphoramide.
Scheme 3
Completion of dictyostatin/discodermolide hybrid 5. a) (R)-CBS, BH3·THF, CH2Cl2, 0 °C, 3 h; b) cat. PPTS, (MeO)2CMe2, 20 °C, 2 h; c) DDQ, CH2Cl2 / pH 7 buffer, 20 °C, 3 h; d) cat. TEMPO, PhI(OAc)2, CH2Cl2, 0 → 20 °C, 1 h; e) NaClO2, NaH2PO4, 2-methyl-2-butene, _t_BuOH / H2O, 20 °C, 4 h; f) 2,4,6-trichlorobenzoylchloride, Et3N, PhMe, 20 °C, 40 min; DMAP, 20 °C, 20 min; g) 3N HCl, MeOH, 0 → 20 °C, 8 h. CBS = Corey-Bakshi-Shibata catalyst; PPTS = pyridinium _para_-toluenesulfonic acid; DDQ = 2,3-dichloro-5,6-dicyano-1,4-benzoquinone; TEMPO = 2,2,6,6-tetramethylpiperidine-1-oxy radical; DMAP = 4-dimethylaminopyridine.
Scheme 4
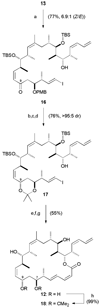
Retrosynthesis of dictyostatin/discodermolide hybrid 12, leading to fragments 13, 14 and 15.
Scheme 5
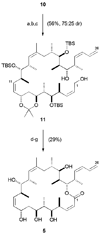
Endgame leading to dictyostatin/discodermolide hybrid 12 and acetonide 18. a) 14, K2CO3, 18-crown-6, PhMe / HMPA, 0 °C, 7 d; b) DDQ, CH2Cl2 / pH 7 buffer, 0 °C, 2.5 h; c) (R)-CBS, BH3· THF, THF, −30 °C, 36 h; d) PPTS, (MeO)2CMe2, CH2Cl2, 0 → 20 °C, 16 h; e) 1. 15, CuTC, NMP, 20 °C, 16 h; 2. KF, MeOH / THF, 20 °C, 90 min; f) 2,4,6-trichlorobenzoylchloride, Et3N, PhMe, 20 °C, 1 h; DMAP, 20 °C, 4 d; g) 3N HCl, MeOH, 0 → 20 °C, 16 h; h) PPTS, (MeO)2CMe2, 0 → 20 °C, 16 h. CuTC = copper(I)-thiophene-2-carboxylate; NMP =_N_-methylpyrrolidinone.
Scheme 6
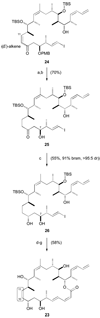
Completion of 10,11-dihydro dictyostatin/discodermolide hybrid 23. a) [PPh3CuH]6, PhMe / H2O, 20 °C, 16 h; b) DDQ, CH2Cl2 / pH 7 buffer, 0 °C, 2.5 h; c) Me4NBH(OAc)3, MeCN / THF, 0 °C, 16 h; d) PPTS, (MeO)2CMe2, CH2Cl2, 0 → 20 °C, 16 h; e) 1. 15, CuTC, NMP, 20 °C, 16 h; 2. KF, MeOH / THF, 20 °C, 2 h; f) 2,4,6-trichlorobenzoylchloride, Et3N, PhMe, 20 °C, 1 h; DMAP, 20 °C, 1 d; g) 3N HCl, MeOH, 0 → 20 °C, 16 h.
Scheme 7
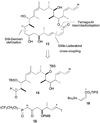
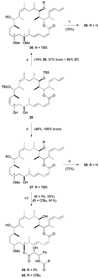
Generation of triple hybrids 31–34. a) PPTS, MeOH / CH2Cl2, 0 → 20 °C, 16 h; b) NaHMDS, THF, −78 °C, 10 min; 29 or 30, −78 → 0 °C, 30 min; c) HF·py, pyridine, THF, 0 → 20 °C, 3 d. NaHMDS = sodium hexamethyldisilazide.
Scheme 8
Synthesis of C9-methoxy analogues 36 and 38–40. a) Me3O·BF4, Proton Sponge, CH2Cl2, 20 °C, 70 min; b) Me3O·BF4, Proton Sponge, CH2Cl2, 20 °C, 45 min; c) 3N HCl, MeOH, 0 → 20 °C, 16 h; d) HF·py, pyridine, THF, 0 → 20 °C, 3 d. e) NaHMDS, THF, −78 °C, 10 min; 29 or 30, −78 → 0 °C, 30 min; f) HF·py, pyridine, THF, 0 → 20 °C, 3 d.
Scheme 9
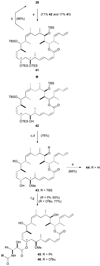
Completion of C7-methoxy analogues 44–46. a) TESOTf, 2,6-lutidine, CH2Cl2, −98 °C, 90 min; b) PPTS, MeOH / CH2Cl2, 0 → 20 °C, 2 h; c) Me3O·BF4, Proton Sponge, CH2Cl2, 20 °C, 90 min; d) PPTS, MeOH / CH2Cl2, 0 → 20 °C, 2 h; e) HF·py, pyridine, THF, 0 → 20 °C, 3 d; f) NaHMDS, THF, −78 °C, 10 min; 29 or 30, −78 → 0 °C; g) HF·py, pyridine, THF, 0 → 20 °C, 3 d. TESOTf = triethylsilyl trifluoromethanesulfonate.
Similar articles
- Total synthesis of a library of designed hybrids of the microtubule-stabilising anticancer agents taxol, discodermolide and dictyostatin.
Paterson I, Naylor GJ, Fujita T, Guzmán E, Wright AE. Paterson I, et al. Chem Commun (Camb). 2010 Jan 14;46(2):261-3. doi: 10.1039/b921237j. Epub 2009 Nov 24. Chem Commun (Camb). 2010. PMID: 20024345 - Development of practical syntheses of the marine anticancer agents discodermolide and dictyostatin.
Florence GJ, Gardner NM, Paterson I. Florence GJ, et al. Nat Prod Rep. 2008 Apr;25(2):342-75. doi: 10.1039/b705661n. Epub 2008 Feb 27. Nat Prod Rep. 2008. PMID: 18389141 Review. - Total synthesis of a potent hybrid of the anticancer natural products dictyostatin and discodermolide.
Paterson I, Naylor GJ, Wright AE. Paterson I, et al. Chem Commun (Camb). 2008 Oct 14;(38):4628-30. doi: 10.1039/b811575c. Epub 2008 Aug 28. Chem Commun (Camb). 2008. PMID: 18815706 - Total synthesis and biological evaluation of potent analogues of dictyostatin: modification of the C2-C6 dienoate region.
Paterson I, Gardner NM, Guzmán E, Wright AE. Paterson I, et al. Bioorg Med Chem Lett. 2008 Dec 1;18(23):6268-72. doi: 10.1016/j.bmcl.2008.09.109. Epub 2008 Oct 11. Bioorg Med Chem Lett. 2008. PMID: 18951787 - The structure activity relationship of discodermolide analogues.
Shaw SJ. Shaw SJ. Mini Rev Med Chem. 2008 Mar;8(3):276-84. doi: 10.2174/138955708783744137. Mini Rev Med Chem. 2008. PMID: 18336347 Review.
Cited by
- Natural products from the Lithistida: a review of the literature since 2000.
Winder PL, Pomponi SA, Wright AE. Winder PL, et al. Mar Drugs. 2011 Dec;9(12):2643-2682. doi: 10.3390/md9122643. Epub 2011 Dec 15. Mar Drugs. 2011. PMID: 22363244 Free PMC article. Review. - Conformation-activity relationships of polyketide natural products.
Larsen EM, Wilson MR, Taylor RE. Larsen EM, et al. Nat Prod Rep. 2015 Aug;32(8):1183-206. doi: 10.1039/c5np00014a. Nat Prod Rep. 2015. PMID: 25974024 Free PMC article. Review. - Total Synthesis and Structure Revision of Halioxepine.
Poock C, Kalesse M. Poock C, et al. Chemistry. 2021 Jan 21;27(5):1615-1619. doi: 10.1002/chem.202004847. Epub 2020 Dec 22. Chemistry. 2021. PMID: 33215739 Free PMC article. - A highly step-economical synthesis of dictyostatin.
Ho S, Bucher C, Leighton JL. Ho S, et al. Angew Chem Int Ed Engl. 2013 Jun 24;52(26):6757-61. doi: 10.1002/anie.201302565. Epub 2013 May 10. Angew Chem Int Ed Engl. 2013. PMID: 23666786 Free PMC article. No abstract available. - Natural products: a continuing source of novel drug leads.
Cragg GM, Newman DJ. Cragg GM, et al. Biochim Biophys Acta. 2013 Jun;1830(6):3670-95. doi: 10.1016/j.bbagen.2013.02.008. Epub 2013 Feb 18. Biochim Biophys Acta. 2013. PMID: 23428572 Free PMC article. Review.
References
- Koehn FE, Carter GT. Nat. Rev. Drug Discovery. 2005;4:206. - PubMed
- Butler MS. Nat. Prod. Rep. 2008;25:475. - PubMed
- Newman DJ, Cragg GM. J. Nat. Prod. 2007;70:461. - PubMed
- Paterson I, Anderson EA. Science. 2005;310:451. - PubMed
- Cragg GML, Kingston DGI, Newman DJ. Anticancer Agents From Natural Products. Boca Raton: Taylor & Francis Group; 2005.
- Nicolaou KC, Dai WM, Guy RK. Angew. Chem. 1994;1994;106:38.
- Angew. Chem. Int. Ed. 1994;33:15.
- Schiff PB, Fant J, Horwitz SB. Nature. 1979;277:665. - PubMed
- Gueritte-Voegelein F, Guenard D, Lavelle F, Le Goff MT, Mangatal L, Potier P. J. Med. Chem. 1991;34:992. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous