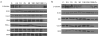Hijacking a biosynthetic pathway yields a glycosyltransferase inhibitor within cells - PubMed (original) (raw)
Hijacking a biosynthetic pathway yields a glycosyltransferase inhibitor within cells
Tracey M Gloster et al. Nat Chem Biol. 2011 Mar.
Abstract
Glycosyltransferases are ubiquitous enzymes that catalyze the assembly of glycoconjugates throughout all kingdoms of nature. A long-standing problem is the rational design of probes that can be used to manipulate glycosyltransferase activity in cells and tissues. Here we describe the rational design and synthesis of a nucleotide sugar analog that inhibits, with high potency both in vitro and in cells, the human glycosyltransferase responsible for the reversible post-translational modification of nucleocytoplasmic proteins with O-linked N-acetylglucosamine residues (O-GlcNAc). We show that the enzymes of the hexosamine biosynthetic pathway can transform, both in vitro and in cells, a synthetic carbohydrate precursor into the nucleotide sugar analog. Treatment of cells with the precursor lowers O-GlcNAc in a targeted manner with a single-digit micromolar EC(50). This approach to inhibition of glycosyltransferases should be applicable to other members of this superfamily of enzymes and enable their manipulation in a biological setting.
Figures
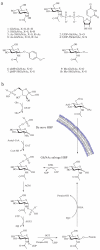
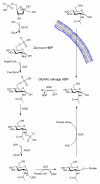
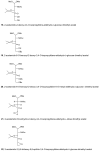
Figure 1. The mammalian hexosamine biosynthetic pathway (HBP), GlcNAc salvage pathway, and the structures of compounds studied here
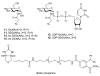
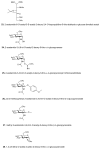
(a) Structures of some of the molecules used in this study (X = O or S as indicated, R = H or Ac as indicated). (b) The end product of the HBP is UDP-GlcNAc (2), the donor substrate used by OGT. The first step in the de novo pathway, where fructose-6-phosphate is converted to glutamine-6-phosphate, is catalyzed by glutamine: fructose-6-phosphate amidotransferase (GFAT). Glutamine-6-phosphate is transformed into GlcNAc-6-phosphate by acetyl-CoA:D-glucosamine-6-phosphate _N_-acetyltransferase (GAT). The GlcNAc salvage pathway recycles cellular GlcNAc (1), which is converted into GlcNAc-6-phosphate by GlcNAc kinase (GNK). GlcNAc-6-phosphate is converted into GlcNAc-1-phosphate by GlcNAc mutase (AGM), and then to the end product, UDP-GlcNAc, by UDP-GlcNAc pyrophosphorylase (AGX1). The endocyclic heteroatom is denoted X (O in nature and S in the synthetic compounds depicted in panel a). 5SGlcNAc (3) is converted via the salvage pathway to generate intracellular UDP-5SGlcNAc (4). Ac-5SGlcNAc (5) is deacetylated by cellular esterases.
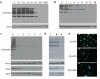
Figure 2. Ac-5SGlcNAc (5) acts in cells to decrease global _O_-GlcNAc levels in a dose and time dependent manner
(a) Western blots of COS-7 cell lysates following Ac-5SGlcNAc (5) administration at different doses (0-1000 μM) for 24 h. Upper panel, probed with anti-_O_-GlcNAc antibody (CTD110.6); lower panel, probed with anti-actin antibody. Densitometry analysis (see Supplementary Figure 6a) yields an EC50 value of 5 μM. (b) Western blots of COS-7 cell lysates following Ac-5SGlcNAc (5) administration at 50 μM for various times. Upper panel, probed with CTD110.6; lower panel, probed with anti-actin antibody. Densitometry analysis (see Supplementary Figure 6e) shows how _O_-GlcNAc levels diminish over time. (c) Western blots of COS-7 cell lysates following Ac-5SGlcNAc (5) administration at 50 μM for different amounts of time. Probed with (from top to bottom) anti-_O_-GlcNAc antibody (CTD110.6), anti-OGA antibody, anti-OGT antibody, and anti-actin antibody. Full versions of the blots are shown in Supplementary Figure 9b. (d) Western blots of COS-7 cell lysates administered specified agents at 50 μM for 24 h; vehicle only (C), Ac-GlcNAc (8) or Ac-5SGlcNAc (5). Probed with (from top to bottom) anti-_O_-GlcNAc antibody (CTD110.6), anti-OGA antibody, anti-OGT antibody, and anti-actin antibody. Full versions of the blots are shown in Supplementary Figure 9a. (e) Immunocytochemistry of COS-7 cells treated with no, 50 μM or 250 μM Ac-5SGlcNAc (5) for 24 hours. Immunoreactivity from anti-_O_-GlcNAc antibody CTD110.6 is shown in green and DAPI (which stains DNA in the nucleus) in blue. The white bar corresponds to a distance of 25 μM.
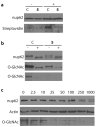
Figure 3. Evaluation of the effects of Ac-5SGlcNAc (5) treatment of cells on the _O_-GlcNAc modification state of nup62
(a) Western blots of immunoprecipitated nup62 from cell lysates following vehicle (C) or 250 μM Ac-5SGlcNAc (5) treatment for 24 h. Following immunoprecipitation, nup62 was incubated with UDP-GalNAz in the absence (−) or presence (+) of GalT1 and chemoselectively labelled. Upper panel, probed with anti-nup62 antibody; lower panel, probed with streptavidin. (b) Western blots of immunoprecipitated nup62 from cell lysates following vehicle (C) or 250 μM Ac-5SGlcNAc (5) treatment for 24 h. Following immunoprecipitation, nup62 was incubated with buffer (−) or with _Bt_GH84 (+) for 2 h to remove _O_-GlcNAc. Upper panel, probed with anti-nup62 antibody; middle panel, probed with anti-_O_-GlcNAc antibody (CTD110.6) (long exposure); bottom panel probed with anti-_O_-GlcNAc antibody (CTD110.6) (short exposure). (c) Western blots of COS-7 cell lysates following Ac-5SGlcNAc (5) administration at different doses (0-1000 μM) for 24 h. Blots are probed with (from top to bottom) anti-nup62 antibody, anti-_O_-GlcNAc antibody (CTD110.6) and anti-actin antibody. Full versions of all of these blots are shown in Supplementary Figure 11.
Figure 4. Metabolic feeding of Ac-5SGlcNAz (14) to cells causes a decrease in _O_-GlcNAc levels, but chemoselecctive ligation demonstrates there is no accumulation of 5SGlcNAz (13) on proteins
(a) Structures of the molecules used or generated during the chemoselective ligation study (X = O or S as indicated, R = H or Ac as indicated). (b) Western blots of COS-7 cell lysates administered specified agents at 50 μM for 24 h; vehicle only (C), Ac-GlcNAz (15) or Ac-5SGlcNAz (14). Cells were harvested and then underwent the Staudinger ligation with biotin phosphine. Blots are probed with (from top to bottom) streptavidin-HRP, anti _O_-GlcNAc (CTD110.6) antibody and anti-actin antibody. (c) Western blot of immunoprecipitated nup62 from cell lysates following vehicle (C), 50 μM Ac-GlcNAz (15) or Ac-5SGlcNAz (14) treatment for 24 h. Following immunoprecipitation, nup62 was incubated with buffer (−) or with _Bt_GH84 (+) for 2 h to remove _O_-GlcNAc, and then underwent the Staudinger ligation with biotin phosphine. Blots are probed with streptavidin-HRP. A full version of this western blot is shown in Supplementary Figure 13.
Figure 5. Ac-5SGlcNAc (5) is converted in cells to generate intracellular UDP-5SGlcNAc (4), causing perturbations in UDP-sugar nucleotide pools
(a) Analysis of UDP-sugar pools of COS-7 cells treated with different concentrations of Ac-5SGlcNAc (0-1000 μM from bottom to top) for 24 h; the CE trace shows absorbance at 254 nm as a function of retention time. Peak A, GDP-Glc (internal standard); peak B, UDP-GlcNAc; peak C, UDP-Glc; peak D, UDP-5SGalNAc; peak E, UDP-5SGlcNAc; peak F, UDP-GalNAc; peak G, UDP-Gal. UDP-5SGlcNAc (4) and UDP-GalNAc co-elute, but the amount of each is estimated using the epimeric ratios determined from the standards (Supplementary Figure 14b). (b) Bar chart showing relative concentrations of UDP-GlcNAc (2), UDP-5SGlcNAc (4), UDP-Gal, UDP-5SGal, UDP-Glc, and UDP-Gal following treatment with 0-1000 μM Ac-5SGlcNAc (5).
Figure 6. Treatment of cells with Ac-5SGlcNAc (5) has no apparent effects on global _N_-glycosylation or _N_-glycosylation of a secreted IgG as evaluated by lectin blot analysis
(a) Western blots of COS-7 cell lysates following Ac-5SGlcNAc (5) administration at different doses (0-1000 μM) for 24 h. C+ denotes untreated cell lysate incubated with PNGase F and C- denotes untreated cell lysate incubated with vehicle. Blots are probed with (from top to bottom) anti-_O_-GlcNAc antibody (CTD110.6), anti-actin antibody, ConA lectin (recognizes α-
d
-mannose, α-
d
-glucose and branched mannose), GNA lectin (recognizes mannose), PHA-L (recognizes complex branched chain oligosaccharide structure), SNA lectin (recognizes NeuAcα(2,6)Gal/GalNAc) and MAA lectin (recognizes NeuAcα(2,3)Gal). Full versions of these blots are shown in Supplementary Figure 15a. (b) Western blots of mouse hybridoma cell lysates (_O_-GlcNAc and actin) and immunoprecipitated mouse hybridoma antibody (IgG, ConA and GNA) following administration of Ac-5SGlcNAc (5) at different doses (0-1000 μM) for 24 h. C+, untreated cell lysate incubated with PNGase F; C-, untreated cell lysate incubated with vehicle. Blots are probed with (from top to bottom) anti-_O_-GlcNAc antibody (CTD110.6), anti-actin antibody, anti-IgG antibody, ConA lectin and GNA lectin. Full versions of these blots are shown in Supplementary Figure 15b.
Figure 7
Figure 8
Figure 9
Figure 10
Figure 11
Figure 13
Figure 14
Figure 15
Similar articles
- Evidence for a Functional O-Linked N-Acetylglucosamine (O-GlcNAc) System in the Thermophilic Bacterium Thermobaculum terrenum.
Ostrowski A, Gundogdu M, Ferenbach AT, Lebedev AA, van Aalten DM. Ostrowski A, et al. J Biol Chem. 2015 Dec 18;290(51):30291-305. doi: 10.1074/jbc.M115.689596. Epub 2015 Oct 21. J Biol Chem. 2015. PMID: 26491011 Free PMC article. - E. coli sabotages the in vivo production of O-linked β-N-acetylglucosamine-modified proteins.
Goodwin OY, Thomasson MS, Lin AJ, Sweeney MM, Macnaughtan MA. Goodwin OY, et al. J Biotechnol. 2013 Dec;168(4):315-23. doi: 10.1016/j.jbiotec.2013.10.008. Epub 2013 Oct 16. J Biotechnol. 2013. PMID: 24140293 - Emerging roles of protein O-GlcNAcylation in cardiovascular diseases: Insights and novel therapeutic targets.
Bolanle IO, Riches-Suman K, Williamson R, Palmer TM. Bolanle IO, et al. Pharmacol Res. 2021 Mar;165:105467. doi: 10.1016/j.phrs.2021.105467. Epub 2021 Jan 27. Pharmacol Res. 2021. PMID: 33515704 Review. - Hexosamine biosynthetic pathway and _O_-GlcNAc cycling of glucose metabolism in brain function and disease.
Kim DY, Park J, Han IO. Kim DY, et al. Am J Physiol Cell Physiol. 2023 Oct 1;325(4):C981-C998. doi: 10.1152/ajpcell.00191.2023. Epub 2023 Aug 21. Am J Physiol Cell Physiol. 2023. PMID: 37602414 Review.
Cited by
- Targeting Selectins and Their Ligands in Cancer.
Natoni A, Macauley MS, O'Dwyer ME. Natoni A, et al. Front Oncol. 2016 Apr 18;6:93. doi: 10.3389/fonc.2016.00093. eCollection 2016. Front Oncol. 2016. PMID: 27148485 Free PMC article. Review. - Synthetic Fluorinated l-Fucose Analogs Inhibit Proliferation of Cancer Cells and Primary Endothelial Cells.
Dai Y, Hartke R, Li C, Yang Q, Liu JO, Wang LX. Dai Y, et al. ACS Chem Biol. 2020 Oct 16;15(10):2662-2672. doi: 10.1021/acschembio.0c00228. Epub 2020 Sep 25. ACS Chem Biol. 2020. PMID: 32930566 Free PMC article. - The roles of O-linked β-N-acetylglucosamine in cardiovascular physiology and disease.
Zachara NE. Zachara NE. Am J Physiol Heart Circ Physiol. 2012 May 15;302(10):H1905-18. doi: 10.1152/ajpheart.00445.2011. Epub 2012 Jan 27. Am J Physiol Heart Circ Physiol. 2012. PMID: 22287582 Free PMC article. Review. - O-GlcNAcylation regulates lysophosphatidic acid-induced cell migration by regulating ERM family proteins.
Song M, Suh PG. Song M, et al. FEBS Open Bio. 2022 Jun;12(6):1220-1229. doi: 10.1002/2211-5463.13404. Epub 2022 Apr 5. FEBS Open Bio. 2022. PMID: 35347892 Free PMC article. - Targeting Protein _O_-GlcNAcylation, a Link between Type 2 Diabetes Mellitus and Inflammatory Disease.
Bolanle IO, Palmer TM. Bolanle IO, et al. Cells. 2022 Feb 17;11(4):705. doi: 10.3390/cells11040705. Cells. 2022. PMID: 35203353 Free PMC article. Review.
References
- Varki A, Lowe JB. Biological roles of glycans. In: Varki A, et al., editors. Essentials of Glycobiology. CSH Press; 2009.
- Platt FM, Neises GR, Dwek RA, Butters TD. N-butyldeoxynojirimycin is a novel inhibitor of glycolipid biosynthesis. J Biol Chem. 1994;269:8362–8365. - PubMed
- Lowe JB. Glycan-dependent leukocyte adhesion and recruitment in inflammation. Curr Opin Cell Biol. 2003;15:531–538. - PubMed
- Granovsky M, et al. Suppression of tumor growth and metastasis in Mgat5-deficient mice. Nat Med. 2000;6:306–312. - PubMed
- Hart GW, Housley MP, Slawson C. Cycling of O-linked beta-N-acetylglucosamine on nucleocytoplasmic proteins. Nature. 2007;446:1017–1022. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources