Deubiquitylase HAUSP stabilizes REST and promotes maintenance of neural progenitor cells - PubMed (original) (raw)
Deubiquitylase HAUSP stabilizes REST and promotes maintenance of neural progenitor cells
Zhi Huang et al. Nat Cell Biol. 2011 Feb.
Abstract
The repressor element 1-silencing transcription factor (REST) functions as a master regulator to maintain neural stem/progenitor cells (NPCs). REST undergoes proteasomal degradation through β-TrCP-mediated ubiquitylation during neuronal differentiation. However, reciprocal mechanisms that stabilize REST in NPCs are undefined. Here we show that the deubiquitylase HAUSP counterbalances REST ubiquitylation and prevents NPC differentiation. HAUSP expression declines concordantly with REST on neuronal differentiation and reciprocally with β-TrCP levels. HAUSP knockdown in NPCs decreases REST and induces differentiation. In contrast, HAUSP overexpression upregulates REST by overriding β-TrCP-mediated ubiquitylation. A consensus site (310-PYSS-313) in human REST is required for HAUSP-mediated REST deubiquitylation. Furthermore, REST overexpression in NPCs rescues the differentiation phenotype induced by HAUSP knockdown. These data demonstrate that HAUSP stabilizes REST through deubiquitylation and antagonizes β-TrCP in regulating REST at the post-translational level. Thus, HAUSP-mediated deubiquitylation represents a critical regulatory mechanism involved in the maintenance of NPCs.
Figures
Figure 1
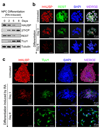
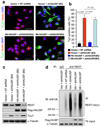
HAUSP and REST protein levels decline coordinately during neuronal differentiation. (a) Immunoblotting of HAUSP, β-TrCP, REST and TUJ1 (a neuronal differentiation marker) during differentiation. 15167 NPCs (neural stem/progenitor cells derived from a fetal brain by Lonza) were induced by all-trans retinoic acid (RA) to undergo cellular differentiation for the indicated times. HAUSP and REST protein levels gradually decreased, while the β-TrCP E3 ubiquitin ligase and the TUJ1 (type III β-tubulin, a REST target gene) levels increased during NPC differentiation. (b) Immunofluorescent staining confirmed that both HAUSP (red) and REST (green) protein levels declined during NPC differentiation. 15167 NPCs were induced by RA to undergo differentiation for the indicated times, then fixed and immunostained with anti-HAUSP and anti-REST specific antibodies. Nuclei were counterstained with DAPI (blue). (c) Immunofluorescent staining showed that HAUSP (red) decreased but the neuronal differentiation marker TUJ1 (green) increased during differentiation. ENStemA NPCs (derived from a human ES cell line by Chemicon/Millipore) were differentiated by RA treatment for the indicated times, fixed and stained with anti-HAUSP and anti-TUJ1 specific antibodies. Nuclei were counterstained with DAPI (blue). Uncropped images of blots are shown in Supplementary Information, Fig. S8.
Figure 2
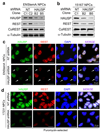
HAUSP knockdown reduces REST protein levels in NPCs. (a, b) Immunoblotting showed that HAUSP knockdown by two distinct shRNAs (B2 and B5) decreased protein levels of REST but not CoREST in ENStemA (a) and 15167 (b) NPCs. NPCs were infected with lentiviruses expressing shHAUSP or non-targeting (NT) control shRNA for 48 hours, whole cell lysates were harvested for immunoblotting with the specific antibodies as indicated. (c, d) Immunofluorescent staining confirmed that HAUSP knockdown reduced REST levels in ENStemA (c) and 17231 (d) NPCs. Cells were cultured and attached on cover glasses coated with BD Matrigel hESC-qualified matrix, infected with lentiviruses expressing shHAUSP or control NT shRNA, treated without (c) or with (d) puromycin to select for infected cells, fixed and immunostained with anti-HAUSP and anti-REST specific antibodies. HAUSP was labeled in green, and REST was labeled in red. Nuclei were counterstained with DAPI (blue). Nuclei with reduced HAUSP and REST proteins are indicated by arrows in c. All Puromycin-selected cells infected with lentiviruses expressing HAUSP-targeting shRNA showed reduced HAUSP and REST protein levels in d. Lentiviral infection efficiency in NPCs with GFP-expressing lentiviruses is shown in Supplementary Information, Fig. S7. Uncropped images of blots are shown in Supplementary Information, Fig. S8.
Figure 3
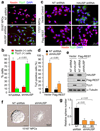
HAUSP knockdown promotes neural differentiation and decreases NPC self-renewal, and REST overexpression rescues the differentiation phenotype induced by HAUSP knockdown. (a) Targeting HAUSP with shRNA promotes neuronal differentiation. 15167 NPCs were infected with lentiviruses expressing shHAUSP (B5 clone) or non-targeting (NT) shRNA for 126 hours and immuno-stained for Nestin (an NPC maker, in red) and TUJ1 (a neuronal differentiation marker, in green). (b) Quantified data from a confirmed that HAUSP knockdown increased neuronal lineage specification. The fraction of cells expressing TUJ1 (green) significantly (p < 0.001) increased and the fraction of cells expressing Nestin (red) decreased after HAUSP knockdown in the NPCs. Data are presented as means ± s.d. [n = 3 (200 cells/experiment)]. (c) Immunofluorescent staining showed that ectopic REST expression rescued the differentiation phenotype induced by HAUSP knockdown. 17231 NPCs were transfected with Flag-REST or vector control, and infected with shHAUSP (B5 clone) or NT shRNA lentiviruses for 126 hours and immuno-stained for Nestin (red) and TUJ1 (green). (d) Quantified data from c indicated that ectopic expression of REST significantly (p < 0.001) attenuated the increased fraction of cells expressing TUJ1 induced by HAUSP knockdown. Data are presented as means ± s.d. [n = 3 (200 cells/experiment)]. (e) Immunoblotting confirmed that ectopic expression of REST repressed the TUJ1 expression induced by HAUSP knockdown. 17231 NPCs were transfected with Flag-REST or vector, and infected with shHAUSP (B5 clone) or NT shRNA lentiviruses for 96 hours, and immunoblotted with specific antibodies against HAUSP, Flag, TUJ1 and α-Tubulin. (f) Neurosphere formation assay showed that HAUSP knockdown reduced NPC self-renewal potential. 15167 NPCs were infected with shHAUSP or NT shRNA lentiviruses and allowed to form neurospheres in serum-free suspension culture. HAUSP knockdown reduced the neurosphere size and induced the attachment of neurosphere on the uncoated dishes. (g) Quantified data from f confirmed that HAUSP knockdown with two specific shRNAs (B2 and B5) significantly (p < 0.001) decreased the number of neurosphere formation of 15167 NPCs. Data are means ± s.d. (n = 3). Uncropped images of blots are shown in Supplementary Information, Fig. S8.
Figure 4
Real-time PCR (RT-PCR) analysis indicated that reduced HAUSP expression by shRNA did not significantly alter REST mRNA expression but increased TUJ1 (a REST target gene) mRNA levels. ENStemA NPCs were targeted with shHAUSP (B5 clone) or control non-targeting (NT) shRNA for 72 hours through lentiviral infection. RNA samples were prepared for RT-PCR analysis with specific primers for HAUSP, REST, Co-REST and TUJ1. HAUSP mRNA was significantly down-regulated but REST mRNA levels were not significantly affected. Data are presented as means ± s.d. (n = 3).
Figure 5
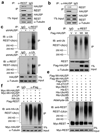
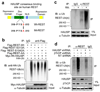
HAUSP mediates REST deubiquitination to regulate REST protein levels. (a, b) Immunoprecipitation (IP) showed that HAUSP and REST interact in NPCs. Cell lysates of ENStemA NPCs were immunoprecipitated with anti-REST (mAb) or anti-HAUSP antibody or IgG control and then immunoblotted with anti-HAUSP and anti-REST (rabbit polyclonal) antibodies. (c) Ubiquitination assays showed that HAUSP knockdown increased REST ubiquitination in NPCs. 17231 NPCs were infected with lentiviruses expressing shHAUSP or non-targeting (NT) shRNA for 48 hours and then treated with the proteasome inhibitor MG132 for 6 hours before harvest for IP. Cell lysates were immunoprecipitated with an anti-REST or anti-Ubiquitin specific antibody or control IgG, and then immunobloted with anti-Ubiquitin or anti-REST specific antibody. Both IP with anti-REST antibody and the reciprocal IP with anti-Ubiquitin antibody confirmed that HAUSP knockdown increased REST poly-ubiquitination. (d) Ectopic expression of wild-type HAUSP (Wt-HAUSP) but not the catalytic dead mutant (223C→S) HAUSP (Mt-HAUSP) reduced REST ubiquitination. 17231 NPCs were transfected with the Flag-tagged Wt-HAUSP (Wt), Mt-HAUSP (Mt) or vector (V) control through lentiviral infection for 36 hours, then treated with the proteasome inhibitor MG132 for 6 hours, and subjected for analysis of REST ubiquitination. Ectopic expression of Wt-HAUSP but not Mt-HAUSP reduced REST ubiquitination in the NPCs. (e) In vitro deubquitination assay showed that the β-TrCP-mediated REST ubiquitination was specifically inhibited by Wt-HAUSP (lane 3) but not by the Mt-HAUSP (a catalytic dead mutant, lane 4) or control deubiquitinase USP1 (lane 5). Flag-Wt-HAUSP, Flag-Mt-HAUSP, Myc-REST, HA-β-TrCP and Flag-USP1 were individually overexpressed in 293T cells, and then purified with the specific antibody or the corresponding tag antibody for this assay. (f) In vivo deubiquitination assay confirmed that the β-TrCP-mediated REST ubiquitination was specifically attenuated by the Wt-HAUSP but not the catalytic dead Mt-HAUSP. 293T cells were transfected with the indicated sets of plasmids for 48 hours, treated with the proteasome inhibitor MG132 for 6 hours and then subjected for analysis of REST ubiquitination in the samples expressing different set of proteins as indicated. Uncropped images of blots are shown in Supplementary Information, Fig. S8.
Figure 6
Neuronal differentiation induced by knockdown of endogenous HAUSP was rescued by ectopic expression of wild-type HAUSP but not the catalytic dead HAUSP mutant. As the B5 shHAUSP clone targets 3’-end non-coding region of endogenous HAUSP mRNA, ectopic expression of the wild-type and mutant HAUSP that lack the 3’-end non-coding region was not affected by the B5 HAUSP shRNA. (a) Immunofluorescent staining showed that ectopic expression of wild-type HAUSP (Wt-HAUSP) but not the catalytic dead mutant HAUSP (Mt-HAUSP) rescued the neuronal differentiation phenotype induced by knockdown of endogenous HAUSP. 17231 NPCs were transfected with Flag-tagged Wt-HAUSP or Mt-HAUSP, or vector control through lentiviral infection, and infected with lentiviruses expressing shHAUSP (B5 clone) or control non-targeting (NT) shRNA for 96 hours and immuno-stained with specific antibodies against Nestin (red) and TUJ1 (green). Ectopic expression of Wt-HAUSP but not the Mt-HAUSP suppressed the expression of neuronal marker TUJ1 that was induced by knockdown of endogenous HAUSP. (b) Quantified data from a indicated that ectopic expression of the Wt-HAUSP but not the Mt-HAUSP in NPCs almost fully rescued the differentiation phenotype induced by knockdown of the endogenous HAUSP. Data are means ± s.d. [n = 4 (200 cells/experiment)]. (c) Immunoblotting validated that ectopic expression of Wt-HAUSP but not the Mt-HAUSP resulted in suppression of TUJ1 expression. 17231 NPCs were transfected with Flag-tagged Wt-HAUSP or Mt-HAUSP, or vector control through lentiviral infection, and infected with lentiviruses expressing shHAUSP (B5 clone) or NT shRNA for 72 hours and immuno-stained with specific antibodies against REST, Flag, TUJ1 and α-Tubulin (loading control). (d) Deubiquitination assay showed that ectopic expression of Wt-HAUSP but not the catalytic dead Mt-HAUSP attenuated the REST ubiquitination induced by knockdown of endogenous HAUSP. 15167 NPCs were transfected with Flag-tagged Wt-HAUSP or Mt-HAUSP, or vector control through lentiviral infection, and infected with lentiviruses expressing shHAUSP (B5 clone) or NT shRNA for 48 hours, treated with the proteasome inhibitor MG132 for 6 hours, immunoprecipitated (IP) with anti-REST specific antibody or the IgG control, and immunobloted with anti-Ubiquitin specific antibody. Uncropped images of blots are shown in Supplementary Information, Fig. S8.
Figure 7
A consensus site of human REST (310-PYSS-313) is required for the HAUSP-mediated REST deubquitination and HAUSP counteracts β-TrCP-mediated REST ubiquitination. (a) Diagram of human REST protein. A consensus sequence (310-PYSS-313) of human REST required for the HAUSP-mediated REST deubiquitination and a critical mutation (S313A) on this site that disrupts the deubiquitination are shown. (b) In vivo deubiquitination assay showed that a critical mutation (S313A) on the consensus sequence (310-PYSS-313) of human REST attenuated the HAUSP-mediated REST deubiquitination (see lanes 2–4), while a similar mutation (S1042A) on another potential site (1039-PQES-1042) did not alter the HAUSP-mediated REST deubiquitination (see lane 3–5). S313A/S1042A double mutations (Flag-REST-AA) and S313A single mutation showed similar effect on the HAUSP-mediated REST deubiquitination (see lane 4–6). 293T cells were transfected with the indicated set of expression plasmids, and treated with the proteasome inhibitor MG132 for 6 hours, and then subjected for analysis of REST ubiquitination. (c) REST ubiquitination increased during neuronal differentiation. 17231 NPCs differentiation was induced by all-trans retinoic acid (RA) for four days, treated with MG132 for 6 hours, and harvested for a ubiquitination assay. Cell lysates from NPCs or differentiated cells were subjected to immunoprecipitation with anti-REST antibody (Rabbit) and immunoblotted with anti-Ubiquitin and the anti-REST antibodies (mAb). (d) Double knockdown analysis confirmed that REST protein is controlled by both β-TrCP-mediated ubiquitination and the HAUSP-mediated deubiquitination in NPCs. 15167 NPCs were treated with RA for only 24 hours to initiate differentiation, and transduced with shHAUSP, shβ-TrCP, both shHAUSP and shβ-TrCP, or non-targeting (NT) shRNA for 36 hours through lentiviral infection, treated with MG132 for 6 hours, and harvested for ubiquitination assessment. HAUSP knockdown alone increased REST ubiquitination (lanes 1, 2), while β-TrCP knockdown alone reduced REST ubiqutination (lanes 1, 3). However, HAUSP and β-TrCP double knockdown restored REST ubiquitination that was reduced by β-TrCP knockdown and abolishes REST ubiquitination induced by HAUSP knockdown (lanes 1–4). Uncropped images of blots are shown in Supplementary Information, Fig. S8.
Figure 8
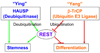
“Ying-Yang” control model of REST protein at the post-translational level. Both HAUSP-mediated deubiquitination (“Ying”) and β-TrCP-mediated ubiquitination (“Yang”) regulate REST protein levels in NPCs. HAUSP deubiquitinase stabilizes REST protein to promote NPC maintenance. In contrast, the β-TrCP E3 ubiquitin ligase mediates REST ubiquitination and degradation to promote neuronal differentiation. The net balance between the β-TrCP-mediated ubiquitination and the HAUSP-mediated deubiquitination controls REST protein levels and determine cellular fate.
Similar articles
- SCFbeta-TRCP controls oncogenic transformation and neural differentiation through REST degradation.
Westbrook TF, Hu G, Ang XL, Mulligan P, Pavlova NN, Liang A, Leng Y, Maehr R, Shi Y, Harper JW, Elledge SJ. Westbrook TF, et al. Nature. 2008 Mar 20;452(7185):370-4. doi: 10.1038/nature06780. Nature. 2008. PMID: 18354483 Free PMC article. - Mutual regulation between β-TRCP mediated REST protein degradation and Kv1.3 expression controls vascular smooth muscle cell phenotype switch.
Ye M, Guo X, Wang H, Wang Y, Qian X, Deng H, Wang W, Yang S, Ni Q, Chen J, Lv L, Zhao Y, Xue G, Li Y, Zhang L. Ye M, et al. Atherosclerosis. 2020 Nov;313:102-110. doi: 10.1016/j.atherosclerosis.2020.08.018. Epub 2020 Aug 29. Atherosclerosis. 2020. PMID: 33038663 - Control of chromosome stability by the beta-TrCP-REST-Mad2 axis.
Guardavaccaro D, Frescas D, Dorrello NV, Peschiaroli A, Multani AS, Cardozo T, Lasorella A, Iavarone A, Chang S, Hernando E, Pagano M. Guardavaccaro D, et al. Nature. 2008 Mar 20;452(7185):365-9. doi: 10.1038/nature06641. Nature. 2008. PMID: 18354482 Free PMC article. - Regulation of NF-κB by ubiquitination and degradation of the IκBs.
Kanarek N, Ben-Neriah Y. Kanarek N, et al. Immunol Rev. 2012 Mar;246(1):77-94. doi: 10.1111/j.1600-065X.2012.01098.x. Immunol Rev. 2012. PMID: 22435548 Review. - ATM-mediated phosphorylations inhibit Mdmx/Mdm2 stabilization by HAUSP in favor of p53 activation.
Meulmeester E, Pereg Y, Shiloh Y, Jochemsen AG. Meulmeester E, et al. Cell Cycle. 2005 Sep;4(9):1166-70. doi: 10.4161/cc.4.9.1981. Epub 2005 Sep 29. Cell Cycle. 2005. PMID: 16082221 Review.
Cited by
- USP7 Is a Master Regulator of Genome Stability.
Valles GJ, Bezsonova I, Woodgate R, Ashton NW. Valles GJ, et al. Front Cell Dev Biol. 2020 Aug 5;8:717. doi: 10.3389/fcell.2020.00717. eCollection 2020. Front Cell Dev Biol. 2020. PMID: 32850836 Free PMC article. Review. - Resequencing of the TMF-1 (TATA Element Modulatory Factor) regulated protein (TRNP1) gene in domestic and wild canids.
Sacco JC, Starr E, Weaver A, Dietz R, Spocter MA. Sacco JC, et al. Canine Med Genet. 2023 Nov 15;10(1):10. doi: 10.1186/s40575-023-00133-0. Canine Med Genet. 2023. PMID: 37968761 Free PMC article. - Organ defects of the Usp7K444R mutant mouse strain indicate the essential role of K63-polyubiquitinated Usp7 in organ formation.
Wu HT, Lin YT, Chew SH, Wu KJ. Wu HT, et al. Biomed J. 2023 Feb;46(1):122-133. doi: 10.1016/j.bj.2022.02.002. Epub 2022 Feb 18. Biomed J. 2023. PMID: 35183794 Free PMC article. - The role of miRNA biogenesis and DDX17 in tumorigenesis and cancer stemness.
Wu KJ. Wu KJ. Biomed J. 2020 Apr;43(2):107-114. doi: 10.1016/j.bj.2020.03.001. Epub 2020 Apr 13. Biomed J. 2020. PMID: 32513392 Free PMC article. Review. - Deubiquitinase USP13 maintains glioblastoma stem cells by antagonizing FBXL14-mediated Myc ubiquitination.
Fang X, Zhou W, Wu Q, Huang Z, Shi Y, Yang K, Chen C, Xie Q, Mack SC, Wang X, Carcaboso AM, Sloan AE, Ouyang G, McLendon RE, Bian XW, Rich JN, Bao S. Fang X, et al. J Exp Med. 2017 Jan;214(1):245-267. doi: 10.1084/jem.20151673. Epub 2016 Dec 6. J Exp Med. 2017. PMID: 27923907 Free PMC article.
References
- Chong JA, et al. REST: a mammalian silencer protein that restricts sodium channel gene expression to neurons. Cell. 1995;80:949–957. - PubMed
- Schoenherr CJ, Anderson DJ. The neuron-restrictive silencer factor (NRSF): a coordinate repressor of multiple neuron-specific genes. Science. 1995;267:1360–1363. - PubMed
- Ballas N, Grunseich C, Lu DD, Speh JC, Mandel G. REST and its corepressors mediate plasticity of neuronal gene chromatin throughout neurogenesis. Cell. 2005;121:645–657. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases