STAT6 activation in ulcerative colitis: a new target for prevention of IL-13-induced colon epithelial cell dysfunction - PubMed (original) (raw)
Comparative Study
. 2011 Nov;17(11):2224-34.
doi: 10.1002/ibd.21628. Epub 2011 Feb 9.
Affiliations
- PMID: 21308881
- PMCID: PMC3120916
- DOI: 10.1002/ibd.21628
Comparative Study
STAT6 activation in ulcerative colitis: a new target for prevention of IL-13-induced colon epithelial cell dysfunction
Michael J Rosen et al. Inflamm Bowel Dis. 2011 Nov.
Abstract
Background: Interleukin 13 (IL-13) is upregulated in ulcerative colitis (UC) and increases colon epithelial permeability by inducing apoptosis and expression of the pore-forming tight junction protein claudin-2. IL-13 induces activation of signal transducer and activator of transcription 6 (STAT6). However, the STAT6 phosphorylation status in patients with UC is unknown, as is the effect of STAT6 inhibition on colonic epithelium exposed to IL-13. The study aims were to determine if mucosal STAT6 phosphorylation is increased in patients with UC, and if STAT6 inhibition attenuates IL-13-induced colon epithelial cell dysfunction.
Methods: Immunohistochemical staining for phosphorylated (p) STAT6 was performed on colonic tissue from newly diagnosed pediatric subjects with UC (early UC) or Crohn's disease (CD), colectomy tissue from adults with UC (advanced UC), and controls. Colon HT-29 and T84 cells were transfected with STAT6 small interfering RNA (siRNA), or treated with suberoylanilide hydroxamic acid (SAHA), a histone deacetylase inhibitor that inhibits STAT6, prior to IL-13 treatment.
Results: The median score for epithelial pSTAT6 was 0 in control subjects, 2 in early UC (versus control P = 0.019), 4 in advanced UC (P = 0.003), and 0 in CD (P = 0.4). Cell transfection with STAT6 siRNA prevented IL-13-induced apoptosis and claudin-2 expression. SAHA inhibited IL-13-induced STAT6 phosphorylation, apoptosis, and claudin-2 expression, and mitigated IL-13-induced reductions in transepithelial resistance.
Conclusions: UC is associated with increased colonic epithelial STAT6 phosphorylation, and STAT6 inhibition prevents IL-13-induced apoptosis and barrier disruption. These data identify STAT6 as a novel target for UC treatment and support further study of SAHA as a therapeutic agent.
Copyright © 2011 Crohn's & Colitis Foundation of America, Inc.
Figures
Figure 1
Increased nuclear pSTAT6 in the colonic epithelium of pediatric subjects at diagnosis with UC. (A) Representative endoscopic biopsy sections from pediatric subjects at diagnosis with UC and normal subjects were stained using anti-pSTAT6 antibody or immunoglobulin control. Magnified inserts (60x) show representative crypts with nuclear staining in the epithelium of the UC tissue. Representative sections stained with anti-pSTAT6 from (B) an adult subject at colostomy for advanced UC and (C) pediatric subjects at diagnosis with CD. (D) Results of pathologist scoring in a fashion blinded to diagnosis for nuclear pSTAT6 in the epithelium and lamina propriety. Bars = 100μm. Pathologist nuclear pSTAT6 scoring system: 0 – no staining, 1 – trace, 2 – <50 cells/hpf, 3 – 50–100 cells/hpf, 4 – >100 cells/hpf. *P ≤ 0.05, **P ≤ 0.01.
Figure 2
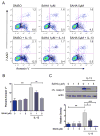
IL-13-induced apoptosis and claudin-2 expression in colon epithelial cells is STAT6-dependent. HT-29 cells were transfected with STAT6 siRNA (SI) or nontargeting (NT) siRNA multiplexes prior to exposure to IL-13 10 ng/ml. (A) STAT6 expression was determined by Western blot analysis. Densitometry was performed to quantify the effectiveness of STAT6 siRNA knockdown. (B) To determine whether STAT6 siRNA transfection reduced STAT6 signaling, cells were exposed to IL-13 for 45 minutes and the lysates were subjected to Western blot analysis for pSTAT6 and total STAT6. The percent of cells undergoing apoptosis was quantified by flow cytometry of Annexin V stained cells. Representative flow cytometry plots (C) and quantification of mean percent Annexin V+ cells from three experiments (D) are shown. Lysates from cells exposed to IL-13 were subjected to Western blot analysis for (E) cleaved caspase-3 and (F) claudin-2. Pooled results of densitometry showing the mean density relative to untreated cells transfected with NT siRNA are shown. Data presented are the results of at least 3 independent experiments. *P ≤ 0.05, **P ≤ 0.01, *** P ≤ 0.001.
Figure 3
SAHA inhibits IL-13-induced STAT6 phosphorylation in colon epithelial cells. HT-29 cells were pre-treated with SAHA at the indicated concentrations for 6 hours followed by exposure to IL-13 (10 ng/ml) for 45 minutes. (A) Whole cell lysates were subjected to Western blot analysis for pSTAT6 and total STAT6. β-actin was assessed as a loading control. Effect of SAHA on IL-13-induced STAT6 phosphorylation was assessed by densitometry. (B) Western blot analysis was performed for IL-13Rα1 and IL-4Rα expression and (C) SOCS1 and SOCS3 expression. (D) HT-29 cells pre-treated with SAHA were exposed to either IL-4 or IL-13 (10 ng/ml) for 45 minutes and cell lysates were subjected to Western blot analysis for pSTAT6. (E) RNA was isolated from cells pre-treated with SAHA followed by exposure to IL-13 for 48 hours and STAT6 expression as measured by real-time PCR. Results are expressed relative to cells treated with DMSO alone. Data presented are the results of at least 3 independent experiments. * P ≤ 0.05, ** P ≤ 0.01, *** P ≤ 0.001.
Figure 4
SAHA inhibits IL-13-induced apoptosis in colon epithelial cells. HT-29 cells were pre-treated with SAHA at the indicated concentrations for 6 hours followed by exposure to IL-13 (10 ng/ml) for 48 hours. The percent of cells undergoing apoptosis was quantified by flow cytometry of Annexin V stained cells. Representative flow cytometry plots (A) and quantification of mean percent Annexin V+ cells from three experiments (B) are shown. (C) Cell lysates were subjected to Western blot analysis for cleaved caspase-3. The results of at least three independent experiments were analyzed by densitometry. *P ≤ 0.05, **P ≤ 0.01, *** P ≤ 0.001.
Figure 5
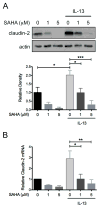
SAHA inhibits IL-13-induced apoptosis claudin-2 expression in colon epithelial cells. HT-29 cells were pre-treated with SAHA at the indicated concentrations for 6 hours followed by exposure to IL-13 (10 ng/ml) for 48 hours. (A) Cell lysates were subjected to Western blot analysis for claudin-2. The results of three independent experiments were analyzed by densitometry. (B) RNA was isolated from cells and STAT6 expression as measured by real-time PCR. Results of three independent experiments are expressed relative to cells treated with DMSO alone. *P ≤ 0.05, **P ≤ 0.01, *** P ≤ 0.001.
Figure 6
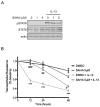
SAHA mitigates IL-13-induced epithelial barrier dysfunction. (A) T84 cells were pre-treated with SAHA at the indicated concentrations for 6 hours followed by exposure to IL-13 10 ng/ml for 45 minutes. Whole cell lysates were subjected to Western blot analysis for pSTAT6 and total STAT6. β-actin was assessed as a loading control. (B) T84 monolayers were cultured on standing membrane inserts, pre-treated with DMSO or SAHA 5 μM for 6 hours followed by exposure to IL-13 (10 ng/ml). TER was measured at the indicated time points and results of four independent experiments are expressed relative to baseline. ***P ≤ 0.001 compared to DMSO; #P ≤ 0.05, ##P ≤ 0.01, ###P ≤ 0.01 compared to DMSO + IL-13.
Similar articles
- Inflammatory cytokines down-regulate the barrier-protective prostasin-matriptase proteolytic cascade early in experimental colitis.
Buzza MS, Johnson TA, Conway GD, Martin EW, Mukhopadhyay S, Shea-Donohue T, Antalis TM. Buzza MS, et al. J Biol Chem. 2017 Jun 30;292(26):10801-10812. doi: 10.1074/jbc.M116.771469. Epub 2017 May 10. J Biol Chem. 2017. PMID: 28490634 Free PMC article. - STAT6 deficiency ameliorates severity of oxazolone colitis by decreasing expression of claudin-2 and Th2-inducing cytokines.
Rosen MJ, Chaturvedi R, Washington MK, Kuhnhein LA, Moore PD, Coggeshall SS, McDonough EM, Weitkamp JH, Singh AB, Coburn LA, Williams CS, Yan F, Van Kaer L, Peebles RS Jr, Wilson KT. Rosen MJ, et al. J Immunol. 2013 Feb 15;190(4):1849-58. doi: 10.4049/jimmunol.1201373. Epub 2013 Jan 9. J Immunol. 2013. PMID: 23303670 Free PMC article. - Non-hematopoietic STAT6 induces epithelial tight junction dysfunction and promotes intestinal inflammation and tumorigenesis.
Lin Y, Li B, Yang X, Liu T, Shi T, Deng B, Zhang Y, Jia L, Jiang Z, He R. Lin Y, et al. Mucosal Immunol. 2019 Nov;12(6):1304-1315. doi: 10.1038/s41385-019-0204-y. Epub 2019 Sep 18. Mucosal Immunol. 2019. PMID: 31534167 - Shift from pStat6 to pStat3 predominance is associated with inflammatory bowel disease-associated dysplasia.
Wick EC, LeBlanc RE, Ortega G, Robinson C, Platz E, Pardoll DM, Iacobuzio-Donahue C, Sears CL. Wick EC, et al. Inflamm Bowel Dis. 2012 Jul;18(7):1267-74. doi: 10.1002/ibd.21908. Epub 2011 Oct 21. Inflamm Bowel Dis. 2012. PMID: 22021169 Free PMC article. - Interleukin-13 is the key effector Th2 cytokine in ulcerative colitis that affects epithelial tight junctions, apoptosis, and cell restitution.
Heller F, Florian P, Bojarski C, Richter J, Christ M, Hillenbrand B, Mankertz J, Gitter AH, Bürgel N, Fromm M, Zeitz M, Fuss I, Strober W, Schulzke JD. Heller F, et al. Gastroenterology. 2005 Aug;129(2):550-64. doi: 10.1016/j.gastro.2005.05.002. Gastroenterology. 2005. PMID: 16083712
Cited by
- Risk of Autoimmunity in EoE and Families: A Population-Based Cohort Study.
Peterson K, Firszt R, Fang J, Wong J, Smith KR, Brady KA. Peterson K, et al. Am J Gastroenterol. 2016 Jul;111(7):926-32. doi: 10.1038/ajg.2016.185. Epub 2016 May 24. Am J Gastroenterol. 2016. PMID: 27215923 - JAK-STAT pathway targeting for the treatment of inflammatory bowel disease.
Salas A, Hernandez-Rocha C, Duijvestein M, Faubion W, McGovern D, Vermeire S, Vetrano S, Vande Casteele N. Salas A, et al. Nat Rev Gastroenterol Hepatol. 2020 Jun;17(6):323-337. doi: 10.1038/s41575-020-0273-0. Epub 2020 Mar 19. Nat Rev Gastroenterol Hepatol. 2020. PMID: 32203403 Review. - Therapeutic activity of an interleukin-4/interleukin-13 dual antagonist on oxazolone-induced colitis in mice.
Kasaian MT, Page KM, Fish S, Brennan A, Cook TA, Moreira K, Zhang M, Jesson M, Marquette K, Agostinelli R, Lee J, Williams CM, Tchistiakova L, Thakker P. Kasaian MT, et al. Immunology. 2014 Nov;143(3):416-27. doi: 10.1111/imm.12319. Immunology. 2014. PMID: 24831554 Free PMC article. - Silencing of IL13RA2 promotes partial epithelial-mesenchymal transition in hepatocellular carcinoma via ERK signaling pathway activation.
Wang M, Yao R, Wang Y. Wang M, et al. FEBS Open Bio. 2020 Feb;10(2):229-236. doi: 10.1002/2211-5463.12774. Epub 2020 Jan 10. FEBS Open Bio. 2020. PMID: 31823484 Free PMC article. - T cell protein tyrosine phosphatase prevents STAT1 induction of claudin-2 expression in intestinal epithelial cells.
Krishnan M, McCole DF. Krishnan M, et al. Ann N Y Acad Sci. 2017 Oct;1405(1):116-130. doi: 10.1111/nyas.13439. Epub 2017 Aug 14. Ann N Y Acad Sci. 2017. PMID: 28804910 Free PMC article.
References
- Xavier RJ, Podolsky DK. Unravelling the pathogenesis of inflammatory bowel disease. Nature. 2007;448:427–434. - PubMed
- Fuss IJ, Neurath M, Boirivant M, et al. Disparate CD4+ lamina propria (LP) lymphokine secretion profiles in inflammatory bowel disease. Crohn's disease LP cells manifest increased secretion of IFN-gamma, whereas ulcerative colitis LP cells manifest increased secretion of IL-5. J Immunol. 1996;157:1261–1270. - PubMed
- Heller F, Florian P, Bojarski C, et al. Interleukin-13 is the key effector Th2 cytokine in ulcerative colitis that affects epithelial tight junctions, apoptosis, and cell restitution. Gastroenterology. 2005;129:550–564. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- P30 DK058404-05/DK/NIDDK NIH HHS/United States
- T32 DK007673/DK/NIDDK NIH HHS/United States
- 2T32DK007673-16/DK/NIDDK NIH HHS/United States
- R01 AT004821/AT/NCCIH NIH HHS/United States
- K01 DK077956-05/DK/NIDDK NIH HHS/United States
- K01DK077956/DK/NIDDK NIH HHS/United States
- P30DK058404/DK/NIDDK NIH HHS/United States
- R01 AT004821-02S1/AT/NCCIH NIH HHS/United States
- 5R01AT004821-03/AT/NCCIH NIH HHS/United States
- T32 DK007673-16/DK/NIDDK NIH HHS/United States
- K01 DK077956/DK/NIDDK NIH HHS/United States
- R03 DK090295/DK/NIDDK NIH HHS/United States
- P30 DK058404/DK/NIDDK NIH HHS/United States
- R01 AT004821-03/AT/NCCIH NIH HHS/United States
- 3R01AT004821-02S1/AT/NCCIH NIH HHS/United States
- R01 DK056008/DK/NIDDK NIH HHS/United States
- R01DK056008/DK/NIDDK NIH HHS/United States
- R01 DK056008-12/DK/NIDDK NIH HHS/United States
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous