Epithelial tissues have varying degrees of susceptibility to Kras(G12D)-initiated tumorigenesis in a mouse model - PubMed (original) (raw)
Epithelial tissues have varying degrees of susceptibility to Kras(G12D)-initiated tumorigenesis in a mouse model
Kevin C Ray et al. PLoS One. 2011.
Abstract
Activating mutations in the Kras gene are commonly found in some but not all epithelial cancers. In order to understand the susceptibility of different epithelial tissues to Kras-induced tumorigenesis, we introduced one of the most common Kras mutations, Kras(G12D), broadly in epithelial tissues. We used a mouse model in which the G12D mutation is placed in the endogenous Kras locus controlled by inducible, Cre-mediated recombination in tissues expressing cytokeratin 19 including the oral cavity, GI tract, lungs, and ducts of the liver, kidney, and the pancreas. Introduction of the Kras(G12D) mutation in adult mouse tissues led to neoplastic changes in some but not all of these tissues. Notably, many hyperplasias, metaplasias and adenomas were observed in the oral cavity, stomach, colon and lungs, suggesting that exposure to products of the outside environment promotes Kras(G12D)-initiated tumorigenesis. However, environmental exposure did not consistently correlate with tumor formation, such as in the small intestine, suggesting that there are also intrinsic differences in susceptibility to Kras activation. The pancreas developed small numbers of mucinous metaplasias with characteristics of early stage pancreatic intraepithelial neoplasms (PanINs), supporting the hypothesis that pancreatic ducts have the potential to give rise pancreatic cancer.
Conflict of interest statement
Competing Interests: The authors have declared that no competing interests exist.
Figures
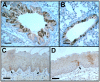
Figure 1. CK19CreERT recombined an EYFP reporter in lung and oral cavity.
Bronchus (A), bronchiole (B), lingual epithelium (C), and buccal epithelium (D) from CK19CreERT; R26REYFP mice immunolabeled for EYFP (brown). Arrows in C and D, labeled cells in basal layer of epithelia. Size bars, 50 µm.
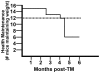
Figure 2. Morbidity analysis: epithelial expression of KrasG12D led to weight loss.
Mice were monitored weekly for weight and other indications of overall health. Number maintaining at least 80% of highest body weight is plotted as a function of time for CK19CreERT; LSL-KrasG12D (solid line) and for littermates also injected with tamoxifen (dashed line). An additional 3 control mice were also injected with tamoxifen but were only followed for 3–5 months as controls for earlier timepoints and did not lose weight during that time (data not shown).
Figure 3. KrasG12D led to squamous papillomas in the oral cavity.
A.H&E staining of papillomas on back of tongue. B. H&E staining of buccal papillomas. C. Immunolabeling for phosphohistone H3 (brown), an M phase marker, revealed that the proliferative zone in papillomas remained in the basal layer. Size bars, 50 µm.
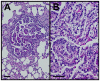
Figure 4. KrasG12D mutation led to adenoma formation in lungs.
A, B. PAS and hematoxylin staining of lung adenomas typical of CK19CreERT; LSL-KrasG12D mice. Size bars, 50 µm.
Figure 5. KrasG12D mutation led to foveolar hyperplasia in the gastric fundus.
A. PAS (pink) showed extensive mucin production deep into affected glands while surrounding normal glands only had staining at the tops of glands. B. Immunolabeling for phosphohistone H3 as an M phase marker. The proliferative zone in affected glands was shifted toward the base of the glands. C. Immunolabeling for TFF2 (brown) showed positive cells near base of affected fundic glands while normal glands had expression in the normal mucous neck region. Arrows, staining in affected glands; arrowheads, staining in normal glands. Size bars, 50 µm.
Figure 6. KrasG12D mutation led to mucinous metaplasia in pancreatic ducts.
H&E staining of a typical lesion in mouse pancreas (A) and its connection to normal ductal epithelium in a nearby serial section (B) with alcian blue staining to denote mucin-producing cells. Arrowhead, junction between nonmucinous and mucinous cells; arrows, junction between cuboidal and columnar cells. (C) Mucinous lesions were all strongly positive for claudin-18 (brown) with highest concentrations along lateral cell borders. Size bars, 50 µm.
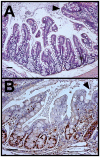
Figure 7. KrasG12D mutation was associated with patches of villus-like structures in the ascending colon.
A. H&E staining shows villus-like architecture in an area of the ascending colon. Normal colonic crypt structure resumes on upper right side of each panel (arrowhead). B. Immunolabeling for Ki67 (brown) indicates that the proliferative zones associated with villus-like structures are similar in cell number to normal colonic crypts. Size bars, 50 µm.
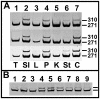
Figure 8. Cre-mediated recombination of the LSL-KrasG12D allele in different tissues.
A. DNA was extracted from the indicated tissues of three different mice and subjected to 35 cycles of PCR that would detect both the wildtype Kras allele (271 bp) and the recombined LSL-KrasG12D allele (310 bp) but not the unrecombined LSL-KrasG12D allele. Tissues examined: 1) tail, 2) small intestine, 3) liver, 4) pancreas, 5) kidney, 6) stomach, and 7) colon. Strong recombined bands were detected for small intestine, pancreas and colon and weaker bands for stomach, kidney and liver, while recombination was never detected in tail DNA. B. The amount of recombination of an unaffected tissue, the small intestine, was compared to the amount in an oral papilloma in which all or nearly all of the epithelium should have a recombined Kras allele. For three different mice, DNA was prepared from total oral tissue (lanes 1–3), isolated papilloma tissue (lanes 4–6) and total small intestine tissue (lanes 7–9) and subjected to 30 cycles of PCR. Little recombination can be detected in total oral tissue while the recombination is abundant in the isolated papilloma (upper band in each lane). The amount of recombination in the small intestine was intermediate between these two populations.
Similar articles
- Oncogenic KRAS Reduces Expression of FGF21 in Acinar Cells to Promote Pancreatic Tumorigenesis in Mice on a High-Fat Diet.
Luo Y, Yang Y, Liu M, Wang D, Wang F, Bi Y, Ji J, Li S, Liu Y, Chen R, Huang H, Wang X, Swidnicka-Siergiejko AK, Janowitz T, Beyaz S, Wang G, Xu S, Bialkowska AB, Luo CK, Pin CL, Liang G, Lu X, Wu M, Shroyer KR, Wolff RA, Plunkett W, Ji B, Li Z, Li E, Li X, Yang VW, Logsdon CD, Abbruzzese JL, Lu W. Luo Y, et al. Gastroenterology. 2019 Nov;157(5):1413-1428.e11. doi: 10.1053/j.gastro.2019.07.030. Epub 2019 Jul 25. Gastroenterology. 2019. PMID: 31352001 Free PMC article. - A Listeria vaccine and depletion of T-regulatory cells activate immunity against early stage pancreatic intraepithelial neoplasms and prolong survival of mice.
Keenan BP, Saenger Y, Kafrouni MI, Leubner A, Lauer P, Maitra A, Rucki AA, Gunderson AJ, Coussens LM, Brockstedt DG, Dubensky TW Jr, Hassan R, Armstrong TD, Jaffee EM. Keenan BP, et al. Gastroenterology. 2014 Jun;146(7):1784-94.e6. doi: 10.1053/j.gastro.2014.02.055. Epub 2014 Mar 6. Gastroenterology. 2014. PMID: 24607504 Free PMC article. - Identification and manipulation of biliary metaplasia in pancreatic tumors.
Delgiorno KE, Hall JC, Takeuchi KK, Pan FC, Halbrook CJ, Washington MK, Olive KP, Spence JR, Sipos B, Wright CV, Wells JM, Crawford HC. Delgiorno KE, et al. Gastroenterology. 2014 Jan;146(1):233-44.e5. doi: 10.1053/j.gastro.2013.08.053. Epub 2013 Aug 30. Gastroenterology. 2014. PMID: 23999170 Free PMC article. - Morphogenesis of pancreatic cancer: role of pancreatic intraepithelial neoplasia (PanINs).
Koorstra JB, Feldmann G, Habbe N, Maitra A. Koorstra JB, et al. Langenbecks Arch Surg. 2008 Jul;393(4):561-70. doi: 10.1007/s00423-008-0282-x. Epub 2008 Feb 19. Langenbecks Arch Surg. 2008. PMID: 18283486 Free PMC article. Review. - Critical role of oncogenic KRAS in pancreatic cancer (Review).
Liu J, Ji S, Liang C, Qin Y, Jin K, Liang D, Xu W, Shi S, Zhang B, Liu L, Liu C, Xu J, Ni Q, Yu X. Liu J, et al. Mol Med Rep. 2016 Jun;13(6):4943-9. doi: 10.3892/mmr.2016.5196. Epub 2016 Apr 27. Mol Med Rep. 2016. PMID: 27121414 Review.
Cited by
- Splicing Factor SRSF1 Promotes Pancreatitis and KRASG12D-Mediated Pancreatic Cancer.
Wan L, Lin KT, Rahman MA, Ishigami Y, Wang Z, Jensen MA, Wilkinson JE, Park Y, Tuveson DA, Krainer AR. Wan L, et al. Cancer Discov. 2023 Jul 7;13(7):1678-1695. doi: 10.1158/2159-8290.CD-22-1013. Cancer Discov. 2023. PMID: 37098965 Free PMC article. - Regulation of Cellular Identity in Cancer.
Roy N, Hebrok M. Roy N, et al. Dev Cell. 2015 Dec 21;35(6):674-84. doi: 10.1016/j.devcel.2015.12.001. Dev Cell. 2015. PMID: 26702828 Free PMC article. Review. - Interaction of the tumor suppressor SMAD4 and WNT signaling in progression to oral squamous cell carcinoma.
Yang J, Lewis JS, Zi J, Andl T, Lee E, Andl CD, Liu Q, Beauchamp RD, Means AL. Yang J, et al. J Pathol. 2024 Sep;264(1):4-16. doi: 10.1002/path.6318. Epub 2024 Jun 26. J Pathol. 2024. PMID: 38922866 - Differential Cell Susceptibilities to KrasG12D in the Setting of Obstructive Chronic Pancreatitis.
Shi C, Pan FC, Kim JN, Washington MK, Padmanabhan C, Meyer CT, Kopp JL, Sander M, Gannon M, Beauchamp RD, Wright CV, Means AL. Shi C, et al. Cell Mol Gastroenterol Hepatol. 2019;8(4):579-594. doi: 10.1016/j.jcmgh.2019.07.001. Epub 2019 Jul 13. Cell Mol Gastroenterol Hepatol. 2019. PMID: 31310834 Free PMC article. - Pathology of pancreatic ductal adenocarcinoma: facts, challenges and future developments.
Esposito I, Konukiewitz B, Schlitter AM, Klöppel G. Esposito I, et al. World J Gastroenterol. 2014 Oct 14;20(38):13833-41. doi: 10.3748/wjg.v20.i38.13833. World J Gastroenterol. 2014. PMID: 25320520 Free PMC article. Review.
References
- Hahn WC, Weinberg RA. Rules for making human tumor cells. N Engl J Med. 2002;347:1593–1603. - PubMed
- Ellis CA, Clark G. The importance of being K-Ras. Cell Signal. 2000;12:425–434. - PubMed
- Luttges J, Reinecke-Luthge A, Mollmann B, Menke MA, Clemens A, et al. Duct changes and K-ras mutations in the disease-free pancreas: analysis of type, age relation and spatial distribution. Virchows Arch. 1999;435:461–468. - PubMed
- Friday BB, Adjei AA. K-ras as a target for cancer therapy. Biochim Biophys Acta. 2005;1756:127–144. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- P30 DK058404/DK/NIDDK NIH HHS/United States
- P30DK058404/DK/NIDDK NIH HHS/United States
- R21CA123061/CA/NCI NIH HHS/United States
- P50CA095103/CA/NCI NIH HHS/United States
- P50 CA095103/CA/NCI NIH HHS/United States
- R01DK065949/DK/NIDDK NIH HHS/United States
- R01 DK065949/DK/NIDDK NIH HHS/United States
- R21 CA123061/CA/NCI NIH HHS/United States
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Miscellaneous