HER2-amplified breast cancer: mechanisms of trastuzumab resistance and novel targeted therapies - PubMed (original) (raw)
Review
HER2-amplified breast cancer: mechanisms of trastuzumab resistance and novel targeted therapies
Devika Gajria et al. Expert Rev Anticancer Ther. 2011 Feb.
Abstract
HER2 amplification is seen in up to 20% of breast cancers and is associated with an aggressive phenotype. Trastuzumab, a monoclonal antibody to HER2, accrues significant clinical benefit in the metastatic and adjuvant settings. However, some patients suffer disease recurrence despite adjuvant trastuzumab therapy, and many patients with metastatic disease do not respond to therapy or develop refractory disease within 1 year of treatment. Given the increased recognition of de novo and acquired resistance to therapy, considerable research has been dedicated to understanding the molecular mechanisms of trastuzumab resistance. Here, we highlight putative models of resistance, including activation of the downstream PI3K-signaling pathway, accumulation of a constitutively active form of HER2, and crosstalk of HER2 with other growth factor receptors. The identification of these specific mechanisms of trastuzumab resistance has provided a rationale for the development of several novel HER2-targeted agents as the mechanisms have largely suggested a continued tumor dependence on HER2 signaling. We explore the emerging data for the treatment of trastuzumab-refractory disease with novel agents including lapatinib, neratinib, pertuzumab, trastuzumab-DM1, HSP90 and PI3K pathway inhibitors, and the future potential for these inhibitors which, if combined with reliable biomarkers of resistance, may ultimately usher in a new era of personalized medicine for this disease.
Figures
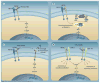
Figure 1. Proposed mechanisms of resistance to trastuzumab
(A) HER2 signal transduction. Activation of the receptor tyrosine kinase occurs by homodimerization or heterodimerization with other HER family members. Activated HER2 initiates downstream signaling through the PI3K–AKT–mTOR pathway, promoting cell proliferation and survival. (B) Downstream activation of the PI3K pathway. PI3K is composed of an 85-kD regulatory subunit and a 110-kD catalytic subunit (PIK3CA), and upon subunit catalyzes phosphorylation of phosphatidylinositol bisphosphate at the membrane (PIP2) to phosphatidylinositol triphosphate promotes membrane localization and activation of downstream effector proteins such as AKT that stimulate cell proliferation. (PIP3). PIP3 or loss of PTEN result PTEN is a negative regulator of PI3K signaling that dephosphorylates PIP3 into PIP2. Activating mutations in PIK3CA in constitutive activation of the PI3K pathway and clinical resistance to trastuzumab therapy. (C) Accumulation of p95-HER2. The constitutively active truncated form of HER2, p95-HER2, lacks the trastuzumab-binding site. The intracellular kinase downstream signaling in the presence of trastuzumab, leading to increased cell proliferation. (D) Increased signaling from alternative receptors. Overexpression or activation of other receptors may drive growth factor signaling, either through trastuzumab-insensitive dimers with HER2 or in a HER2-independent fashion.
Figure 2. Sites of action of novel agents for HER2-amplified breast cancer
(A) Lapatinib is a dual EGF receptor (EGFR)/HER2 tyrosine kinase inhibitor approved for use in trastuzumab-refractory patients. Neratinib is an irreversible tyrosine kinase inhibitor of EGFR/HER2. (B) Pertuzumab, a monoclonal antibody to HER2, binds to HER2 at a distinct epitope from where trastuzumab binds and prevents ligand-induced heterodimerization with HER3. Dysregulated activation of the PI3K–AKT–mTOR pathway can mediate trastuzumab resistance, and molecular therapies aimed at directly inhibiting PI3K, AKT and mTOR are therefore in development. (C) HSP90 inhibitors promote HER2 degradation by blocking the activity of HSP90, a chaperone protein that protects HER2 from proteasomal degradation. (D) TDM-1, the antibody–drug conjugate of trastuzumab and maytansine, allows for delivery of a potent microtubule inhibitor selectively into HER2-overexpressing cells.
Similar articles
- Novel targeted therapies to overcome trastuzumab resistance in HER2-overexpressing metastatic breast cancer.
Huang Y, Fu P, Fan W. Huang Y, et al. Curr Drug Targets. 2013 Jul;14(8):889-98. doi: 10.2174/13894501113149990161. Curr Drug Targets. 2013. PMID: 23531110 Review. - Beyond trastuzumab and lapatinib: new options for HER2-positive breast cancer.
Zardavas D, Cameron D, Krop I, Piccart M. Zardavas D, et al. Am Soc Clin Oncol Educ Book. 2013. doi: 10.14694/EdBook_AM.2013.33.e2. Am Soc Clin Oncol Educ Book. 2013. PMID: 23714441 Review. - Autophagy-related gene 12 (ATG12) is a novel determinant of primary resistance to HER2-targeted therapies: utility of transcriptome analysis of the autophagy interactome to guide breast cancer treatment.
Cufí S, Vazquez-Martin A, Oliveras-Ferraros C, Corominas-Faja B, Urruticoechea A, Martin-Castillo B, Menendez JA. Cufí S, et al. Oncotarget. 2012 Dec;3(12):1600-14. doi: 10.18632/oncotarget.742. Oncotarget. 2012. PMID: 23307622 Free PMC article. - Evolving strategies for overcoming resistance to HER2-directed therapy: targeting the PI3K/Akt/mTOR pathway.
Nahta R, O'Regan RM. Nahta R, et al. Clin Breast Cancer. 2010 Nov;10 Suppl 3:S72-8. doi: 10.3816/CBC.2010.s.015. Clin Breast Cancer. 2010. PMID: 21115425 Review. - A preclinical evaluation of the PI3K alpha/delta dominant inhibitor BAY 80-6946 in HER2-positive breast cancer models with acquired resistance to the HER2-targeted therapies trastuzumab and lapatinib.
Elster N, Cremona M, Morgan C, Toomey S, Carr A, O'Grady A, Hennessy BT, Eustace AJ. Elster N, et al. Breast Cancer Res Treat. 2015 Jan;149(2):373-83. doi: 10.1007/s10549-014-3239-5. Epub 2014 Dec 21. Breast Cancer Res Treat. 2015. PMID: 25528022
Cited by
- Molecular Testing in Stage 4 Stomach Cancer in India: A Single-Centre Experience.
Anand R, Rauthan A, Patil P, Murthy NY. Anand R, et al. Cureus. 2023 Nov 25;15(11):e49412. doi: 10.7759/cureus.49412. eCollection 2023 Nov. Cureus. 2023. PMID: 38024069 Free PMC article. - Continuous requirement of ErbB2 kinase activity for loss of cell polarity and lumen formation in a novel ErbB2/Neu-driven murine cell line model of metastatic breast cancer.
Ortega-Cava CF, Raja SM, Laiq Z, Bailey TA, Luan H, Mohapatra B, Williams SH, Ericsson AC, Goswami R, Dimri M, Duan L, Band V, Naramura M, Band H. Ortega-Cava CF, et al. J Carcinog. 2011;10:29. doi: 10.4103/1477-3163.90443. Epub 2011 Nov 30. J Carcinog. 2011. PMID: 22190871 Free PMC article. - Comprehensive Molecular Characterization of Salivary Duct Carcinoma Reveals Actionable Targets and Similarity to Apocrine Breast Cancer.
Dalin MG, Desrichard A, Katabi N, Makarov V, Walsh LA, Lee KW, Wang Q, Armenia J, West L, Dogan S, Wang L, Ramaswami D, Ho AL, Ganly I, Solit DB, Berger MF, Schultz ND, Reis-Filho JS, Chan TA, Morris LG. Dalin MG, et al. Clin Cancer Res. 2016 Sep 15;22(18):4623-33. doi: 10.1158/1078-0432.CCR-16-0637. Epub 2016 Apr 21. Clin Cancer Res. 2016. PMID: 27103403 Free PMC article. - Personalized Risk-Based Screening Design for Comparative Two-Arm Group Sequential Clinical Trials.
Park Y. Park Y. J Pers Med. 2022 Mar 12;12(3):448. doi: 10.3390/jpm12030448. J Pers Med. 2022. PMID: 35330448 Free PMC article. - Investigating intratumour heterogeneity by single-cell sequencing.
Ren SC, Qu M, Sun YH. Ren SC, et al. Asian J Androl. 2013 Nov;15(6):729-34. doi: 10.1038/aja.2013.106. Epub 2013 Oct 21. Asian J Androl. 2013. PMID: 24141534 Free PMC article. Review.
References
- Slamon DJ, Clark GM, Wong SG, Levin WJ, Ullrich A, McGuire WL. Human breast cancer: correlation of relapse and survival with amplification of the HER-2/Neu oncogene. Science. 1987;235:177–182. - PubMed
- Andrulis IL, Bull SB, Blackstein ME, et al. Neu/erbB-2 amplification identifies a poor-prognosis group of women with node-negative breast cancer. Toronto Breast Cancer Study Group. J Clin Oncol. 1998;16:1340–1349. - PubMed
- Sjogren S, Inganas M, Lindgren A, Holmberg L, Bergh J. Prognostic and predictive value of c-erbB-2 overexpression in primary breast cancer, alone and in combination with other prognostic markers. J Clin Oncol. 1998;16:462–469. - PubMed
- Yarden Y, Sliwkowski MX. Untangling the ErbB signalling network. Nat Rev. 2001;2:127–137. - PubMed
- Yarden Y. The EGFR family and its ligands in human cancer. signalling mechanisms and therapeutic opportunities. Eur J Cancer. 2001;37(Suppl 4):S3–S8. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
Miscellaneous