FUS transgenic rats develop the phenotypes of amyotrophic lateral sclerosis and frontotemporal lobar degeneration - PubMed (original) (raw)
FUS transgenic rats develop the phenotypes of amyotrophic lateral sclerosis and frontotemporal lobar degeneration
Cao Huang et al. PLoS Genet. 2011 Mar.
Abstract
Fused in Sarcoma (FUS) proteinopathy is a feature of frontotemporal lobar dementia (FTLD), and mutation of the fus gene segregates with FTLD and amyotrophic lateral sclerosis (ALS). To study the consequences of mutation in the fus gene, we created transgenic rats expressing the human fus gene with or without mutation. Overexpression of a mutant (R521C substitution), but not normal, human FUS induced progressive paralysis resembling ALS. Mutant FUS transgenic rats developed progressive paralysis secondary to degeneration of motor axons and displayed a substantial loss of neurons in the cortex and hippocampus. This neuronal loss was accompanied by ubiquitin aggregation and glial reaction. While transgenic rats that overexpressed the wild-type human FUS were asymptomatic at young ages, they showed a deficit in spatial learning and memory and a significant loss of cortical and hippocampal neurons at advanced ages. These results suggest that mutant FUS is more toxic to neurons than normal FUS and that increased expression of normal FUS is sufficient to induce neuron death. Our FUS transgenic rats reproduced some phenotypes of ALS and FTLD and will provide a useful model for mechanistic studies of FUS-related diseases.
Conflict of interest statement
The authors declare no conflict of interest.
Figures
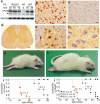
Figure 1. Progressive paralysis in transgenic rats overexpressing a mutant human FUS.
(A) Immunoblotting showed expression of human FUS in normal (WT) and mutant (R521) fus transgenic rats, but not in nontransgenic controls (NT). Each lane was loaded with 30 µg of total protein in the lysates of spinal cord. The blotting membrane was probed with an antibody to human FUS (made in-house) and then with an antibody against GAPDH after stripping. The number of transgenic lines corresponds to the number of FUS transgene copies. * indicates a non-specific band. (B–F) Immunohistochemistry detected expression of human FUS in the cortex (B) and spinal cord (D, E) of FUS transgenic rats (line 16), but not in tissues of nontransgenic littermates (C, cortex; F, spinal cord). Expression of human FUS in lumbar spinal cord profiled at a low magnification (D). Human FUS was diffusely located in the cytoplasm of motor neurons in the ventral horn (E). Scale bars: B–C and E–F: 20 µm; D, 100 µm. (G, H) Representative photographs of male transgenic rats of line 22 (G, 30 days of age) and line 16 (H, 60 days of age) at paralysis stages. (I, J) Graphs show the probability of disease onset (I) and survival (J) in FUS transgenic rats. Disease onset was defined as an unrecoverable reduction in the grip strength of fore or hind paws. Rats were euthanized and counted as dead when two or more legs were paralyzed or body weight was reduced by 30%. All FUS transgenic rats carried the CAG-tTA transgene. Breeding female rats were given Dox in their drinking water (50 µg/ml) until delivery. Each group contained 13 to 16 rats.
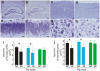
Figure 2. Motor neuron degeneration accompanied by denervation atrophy of skeletal muscle.
(A–C) Cresyl violet staining of motor neurons in the L3 ventral horn of mutant FUS transgenic (C, line 16), age-matched nontransgenic (A), and normal FUS transgenic (B, line 20) rats. (D, E) Bielschowsky silver staining detected degenerating neurons (arrows) in the spinal cord of mutant FUS transgenic rats at paralysis stages (E, line 16), but not in that of age-matched normal FUS transgenic rats (D, line 20). Note that degenerating neurons were rare in the mutant FUS transgenic rats even at paralysis stages. (F–K) Toluidine blue staining of axons of the dorsal corticospinal tract (F, G), L3 ventral roots (H, I), and L3 dorsal roots (J, K). Degenerating axons were seen in paralyzed mutant FUS transgenic rats of line 16 (G, I, K), but not in age-matched normal FUS transgenic rats of line 20 (F, G, J). (L, M) Electron microscopy of the motor axons in the L3 ventral roots of paralyzed mutant FUS (M, line 16) and age-matched normal FUS transgenic rats (L, line 20). Arrows point to degenerating axons. (N, O) H&E staining showed group atrophy (arrows) of the gastrocnemius muscle in paralyzed mutant FUS (O), but not in age-matched normal FUS (N) transgenic rats. (P) Electromyography of gastrocnemius muscles revealed fibrillation (arrows) and fasciculation (arrow head) potentials in a mutant FUS transgenic rat (R521C), but not in its nontransgenic littermate (NT). (Q) Confocal microscopy of the focal structures of neuromuscular junctions (NMJ) in gastrocnemius muscles. Compared to the NMJ of a nontransgenic littermate (NT), the NMJ of a mutant FUS transgenic rat (R521C) was denervated. While axon terminals were visualized by immunostaining for synaptophysin (SYN) and neurofilament (NF), postsynaptic nicotinic receptors were visualized with Alexa fluor 555-conjugated α-bungarotoxin (α-BTX). (R) Quantification of NMJ revealed a reduction of intact NMJ in the mutant FUS (lines 16 and 22), but not in the normal FUS (line 20), transgenic rats. Twenty NMJs were examined for each animal (5-6 rats per genotype). *p<0.05, transgenic rats vs. nontransgenic rats. (S) Stereological cell counting revealed the number of large neurons (>25 µm in diameter) in the ventral horn of L3 spinal segments taken from nontransgenic (NT) or transgenic (TG) rats. Data are presented as means ± SD (n = 9–11). Scale bars: A–E, 50 µm; F–K, 20 µm; L, M, 5 µm; N, O, 30 µm.
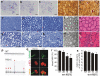
Figure 3. Loss of neurons in the cortex and hippocampus of mutant FUS transgenic rats.
(A–C) Cresyl violet staining of hippocampus (A–B and E–F) and cortex (C–D and G–H) showed faint staining in the tissues of mutant FUS transgenic rats at disease end-stages (B, D, F, and H) compared to age-matched nontransgenic rats (A, C, E, and G). Scale bars: A–B, 400 µm; C–D, 50 µm; and E–H, 20 µm. (I, J) Stereological cell counting revealed a loss of neurons in the cortex (I) and dentate gyrus (J) of mutant FUS transgenic rats (lines 16 and 20). The CAG-tTA single transgenic rats and nontransgenic rats were combined as control rats (NT) because no difference was observed between these rats. Mutant FUS transgenic rats were killed at disease end-stages and paired control rats were killed at matched ages. Normal FUS transgenic rats and their paired controls were killed at 70 days of age, by which time mutant FUS transgenic rats had reached disease end-stages. Data are presented as means ± SD (n = 5–7). *p<0.05.
Figure 4. Neuron loss accompanied by ubiquitin aggregation in aged rats overexpressing the normal human fus gene.
(A–C) Double-fluorescence staining for human FUS (A, red) and the neuronal marker NeuN (B, green) in the frontal cortex of normal human FUS transgenic rats (line 20). Most FUS-positive cells expressed NeuN, but some did not (C). (D–I) Immunofluorescence staining revealed ubiquitin aggregation in aged (G–I; 1 year of age), but not young (D–F; 3 months of age), normal FUS transgenic rats. Coronal sections of frontal cortex were immunostained with antibodies to ubiquitin (D, G: green) and human FUS (E, H: red). Scale bars: A–C, 100 µm; D–I, 20 µm. (J, K) Barnes maze analysis revealed spatial learning deficits in normal FUS transgenic rats of line 20 at advanced ages. One year old transgenic rats (TG) and their nontransgenic littermates (NT) were tested in a Barnes maze and time spent to locate the fixed escape hole (latency) and the number of errors made before escaping were recorded. (L, M) Stereological cell counting revealed a loss of neurons in the cortex (J) and dentate gyrus (K) of normal FUS transgenic rats at advanced ages (line 20). Coronal sections of one hemisphere were stained with Cresyl violet and the number of neurons in the frontal cortex and dentate gyrus was estimated by stereological cell counting. Normal FUS transgenic rats and their nontransgenic controls were killed at the age of 1 year. Data are presented as means ± SD (n = 5). * p<0.05.
Figure 5. Accumulation of ubiquitin in mutant FUS transgenic rats.
(A–L) Double-fluorescence staining revealed accumulation of ubiquitin in the cortex (D–F) and spinal cord (J–L) of the mutant FUS transgenic rats (line 16) at paralysis stages, but not in tissues of the age-matched normal FUS transgenic rats (line 20) (A–C: cortex; G–I: spinal cord). Coronal sections of the frontal cortex and traverse sections of the lumbar cord were immunostained with antibodies to ubiquitin (green) and human FUS (red). Scale bars: A–F, 20 µm.
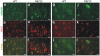
Figure 6. Proliferation of astrocytes and microglia in mutant FUS transgenic rats.
(A–K) Immunofluorescence staining for the microglia marker Iba-1 (A–F) and the astrocyte marker GFAP (H–K) in the mutant FUS transgenic rats of line 16 at the paralysis stage (R521C) and in their nontransgenic age-matched littermates (NT). Coronal sections of the frontal cortex and traverse sections of the lumbar spinal cord were immunostained with antibodies to Iba-1 (red) or GFAP (green). Scale bars: 100 µm.
Similar articles
- Entorhinal cortical neurons are the primary targets of FUS mislocalization and ubiquitin aggregation in FUS transgenic rats.
Huang C, Tong J, Bi F, Wu Q, Huang B, Zhou H, Xia XG. Huang C, et al. Hum Mol Genet. 2012 Nov 1;21(21):4602-14. doi: 10.1093/hmg/dds299. Epub 2012 Jul 23. Hum Mol Genet. 2012. PMID: 22833456 Free PMC article. - Frontotemporal dementia-like disease progression elicited by seeded aggregation and spread of FUS.
Vazquez-Sanchez S, Tilkin B, Gasset-Rosa F, Zhang S, Piol D, McAlonis-Downes M, Artates J, Govea-Perez N, Verresen Y, Guo L, Cleveland DW, Shorter J, Da Cruz S. Vazquez-Sanchez S, et al. Mol Neurodegener. 2024 Jun 11;19(1):46. doi: 10.1186/s13024-024-00737-5. Mol Neurodegener. 2024. PMID: 38862967 Free PMC article. - Dendritic Homeostasis Disruption in a Novel Frontotemporal Dementia Mouse Model Expressing Cytoplasmic Fused in Sarcoma.
Shiihashi G, Ito D, Arai I, Kobayashi Y, Hayashi K, Otsuka S, Nakajima K, Yuzaki M, Itohara S, Suzuki N. Shiihashi G, et al. EBioMedicine. 2017 Oct;24:102-115. doi: 10.1016/j.ebiom.2017.09.005. Epub 2017 Sep 9. EBioMedicine. 2017. PMID: 28928015 Free PMC article. - Pathogenesis of FUS-associated ALS and FTD: insights from rodent models.
Nolan M, Talbot K, Ansorge O. Nolan M, et al. Acta Neuropathol Commun. 2016 Sep 6;4(1):99. doi: 10.1186/s40478-016-0358-8. Acta Neuropathol Commun. 2016. PMID: 27600654 Free PMC article. Review. - Frontotemporal lobar degeneration and amyotrophic lateral sclerosis: molecular similarities and differences.
Neumann M. Neumann M. Rev Neurol (Paris). 2013 Oct;169(10):793-8. doi: 10.1016/j.neurol.2013.07.019. Epub 2013 Sep 5. Rev Neurol (Paris). 2013. PMID: 24011641 Review.
Cited by
- Improvement of Oxidative Stress and Mitochondrial Dysfunction by _β_-Caryophyllene: A Focus on the Nervous System.
Ullah H, Di Minno A, Santarcangelo C, Khan H, Daglia M. Ullah H, et al. Antioxidants (Basel). 2021 Apr 1;10(4):546. doi: 10.3390/antiox10040546. Antioxidants (Basel). 2021. PMID: 33915950 Free PMC article. Review. - Amelioration of toxicity in neuronal models of amyotrophic lateral sclerosis by hUPF1.
Barmada SJ, Ju S, Arjun A, Batarse A, Archbold HC, Peisach D, Li X, Zhang Y, Tank EM, Qiu H, Huang EJ, Ringe D, Petsko GA, Finkbeiner S. Barmada SJ, et al. Proc Natl Acad Sci U S A. 2015 Jun 23;112(25):7821-6. doi: 10.1073/pnas.1509744112. Epub 2015 Jun 8. Proc Natl Acad Sci U S A. 2015. PMID: 26056265 Free PMC article. - AAV2 mediated retrograde transduction of corticospinal motor neurons reveals initial and selective apical dendrite degeneration in ALS.
Jara JH, Villa SR, Khan NA, Bohn MC, Ozdinler PH. Jara JH, et al. Neurobiol Dis. 2012 Aug;47(2):174-83. doi: 10.1016/j.nbd.2012.03.036. Epub 2012 Apr 11. Neurobiol Dis. 2012. PMID: 22521461 Free PMC article. - Defects in synapse structure and function precede motor neuron degeneration in Drosophila models of FUS-related ALS.
Shahidullah M, Le Marchand SJ, Fei H, Zhang J, Pandey UB, Dalva MB, Pasinelli P, Levitan IB. Shahidullah M, et al. J Neurosci. 2013 Dec 11;33(50):19590-8. doi: 10.1523/JNEUROSCI.3396-13.2013. J Neurosci. 2013. PMID: 24336723 Free PMC article. - Dysfunction of RNA/RNA-Binding Proteins in ALS Astrocytes and Microglia.
Rossi S, Cozzolino M. Rossi S, et al. Cells. 2021 Nov 3;10(11):3005. doi: 10.3390/cells10113005. Cells. 2021. PMID: 34831228 Free PMC article. Review.
References
- Wang J, Slunt H, Gonzales V, Fromholt D, Coonfield M, et al. Copper-binding-site-null SOD1 causes ALS in transgenic mice: aggregates of non-native SOD1 delineate a common feature. Hum Mol Genet. 2003;12:2753–2764. - PubMed
- Lillo P, Hodges JR. Frontotemporal dementia and motor neurone disease: Overlapping clinic-pathological disorders. Journal of Clinical Neuroscience. 2009;16:1131–1135. - PubMed
- Boillee S. Onset and Progression in Inherited ALS Determined by Motor Neurons and Microglia. Science. 2006;312:1389–1392. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- R21 NS072696/NS/NINDS NIH HHS/United States
- R01 NS072113/NS/NINDS NIH HHS/United States
- NS072113/NS/NINDS NIH HHS/United States
- R21 NS072696-01/NS/NINDS NIH HHS/United States
- NS072696/NS/NINDS NIH HHS/United States
- R21 NS072696-02/NS/NINDS NIH HHS/United States
- R01 NS072113-02/NS/NINDS NIH HHS/United States
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous