HGF-independent potentiation of EGFR action by c-Met - PubMed (original) (raw)
HGF-independent potentiation of EGFR action by c-Met
A M Dulak et al. Oncogene. 2011.
Abstract
The c-Met receptor is a potential therapeutic target for non-small cell lung cancer (NSCLC). Signaling interactions between c-Met and the mutant epidermal growth factor receptor (EGFR) have been studied extensively, but signaling intermediates and biological consequences of lateral signaling to c-Met in EGFR wild-type tumors are minimally understood. Our observations indicate that delayed c-Met activation in NSCLC cell lines is initiated by wild-type EGFR, the receptor most often found in NSCLC tumors. EGFR ligands induce accumulation of activated c-Met, which begins at 8 h and continues for 48 h. This effect is accompanied by an increase in c-Met expression and phosphorylation of critical c-Met tyrosine residues without activation of mitogen-activated protein kinase (MAPK) or Akt. Gene transcription is required for delayed c-Met activation; however, phosphorylation of c-Met by EGFR occurs without production of hepatocyte growth factor (HGF) or another secreted factor, supporting a ligand-independent mechanism. Lateral signaling is blocked by two selective c-Met tyrosine kinase inhibitors (TKIs), PF2341066 and SU11274, or with gefitinib, an EGFR TKI, suggesting kinase activity of both receptors is required for this effect. Prolonged c-Src phosphorylation is observed, and c-Src pathway is essential for EGFR to c-Met communication. Pretreatment with pan-Src family kinase inhibitors, PP2 and dasatinib, abolishes delayed c-Met phosphorylation. A c-Src dominant-negative construct reduces EGF-induced c-Met phosphorylation compared with control, further confirming a c-Src requirement. Inhibition of c-Met with PF2341066 and siRNA decreases EGF-induced phenotypes of invasion by ~86% and motility by ~81%, suggesting that a novel form of c-Met activation is utilized by EGFR to maximize these biological effects. Combined targeting of c-Met and EGFR leads to increased xenograft antitumor activity, demonstrating that inhibition of downstream and lateral signaling from the EGFR-c-Src-c-Met axis might be effective in treatment of NSCLC.
Conflict of interest statement
Conflict of Interest
The authors declare no conflict of interest.
Figures
Figure 1
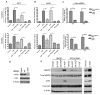
EGFR ligands induce c-Met activation. (A and B) 201T cells were serum-deprived for 2 days prior to stimulation with EGF and TGF-α (10 nM) for 0–48 h followed by immunoprecipitation (IP) with c-Met and western blotting with pY99, total c-Met, and β-Actin antibodies. (C) Specific c-Met phosphorylation sites were measured by c-Met IP then immunoblotting with phospho-c-Met antibodies: Y1003, Y1234/35, Y1349, and Y1365. All blots were stripped and reprobed with a total c-Met antibody to confirm equal loading. (D) 201T cells were serum starved 2 days, pretreated with actinomycin D (0.01 mg/mL), and treated with either EGF (10 nM) for 24 h or HGF (10 ng/mL) for 5 min. Cell lysates were analyzed for c-Met tyrosine phosphorylation and expression.
Figure 2
EGFR and c-Met kinase activity is required for EGF-induced c-Met phosphorylation. 201T cells were serum deprived for 2 days, pretreated with indicated inhibitors, and stimulated with EGF (10 nM) for 24 h or HGF (10 ng/mL) for 5 min. Cell lysates were analyzed for c-Met tyrosine phosphorylation and expression. (A) Pretreatment with gefitinib (100 nM, 1 μM, 10μM) occurred for 2 h, and cells were stimulated with respective ligands. (B) After 2 h pretreatment with the c-Met inhibitors, SU11274 (1 μM) or PF2341066 (1 μM), cells were growth factor treated. (C) Cells were pretreated with gefitinib (10 μM) followed by stimulation with either HGF (10 ng/mL) or EGF (10 nM) for 5 min. Cell lysates were immunoprecipitated for c-Met and probed for pY99 and total c-Met. (D) 201T cells were pretreated with either SU11274 or PF2341066 (1 μM) followed by stimulation with EGF (10 nM) for 5 min. Cell lysates were immunoprecipitated for EGFR and probed for pY99 and total EGFR.
Figure 3
C-Src mediates EGFR-induced c-Met phosphorylation. 201T cells were serum deprived for 2 days followed by addition of EGF (10 nM) for 0–48 h. (A) Cell lysates were immunoprecipitated for total c-Src and analyzed for phospho-c-Src (Y418). (B) 201T cells were pretreated for 2 h with SFK inhibitors PP2 (500 nM) or dasatinib (50 nM) prior to 24 h EGF (10 nM) or 5 min HGF (10 ng/mL) stimulation, or were stably transfected with either a dominant-negative c-Src construct (DN-Src) or empty vector (EV) and stimulated with EGF. Cell lysates were prepared and analyzed for phospho- and total c-Met levels. (C) A549 and 201T cells were pretreated for 2 h with PP2 (500 nM) prior to 24 h EGF stimulation, mRNA was harvested, and subjected to c-Met quantitative RT-PCR using β-Gus as an internal control.
Figure 4
EGFR activation of c-Met does not require HGF production or secretion. A549 and 201T cells were serum starved 2 days prior to stimulation with EGF (10 nM). (A) Tissue culture media was harvested for 0–24 h time points and subjected to HGF ELISA with fibroblast-conditioned media (FCM) as a positive control. (B) Cells were treated, mRNA was harvested, and subjected to HGF quantitative RT-PCR using β-Gus as an internal control. Normal lung fibroblast (NLF) mRNA was utilized as a positive control while the breast cancer cell line, MCF-7, was used as a negative control for HGF expression. (C) EGF (10 nM) or HGF (10 ng/mL) was pretreated for 2 h with HGF neutralizing antibody or IgG control (300 ng/mL) prior to addition to 201T cells for 24 h. Cell lysates were prepared and analyzed for phospho- and total c-Met levels. (D) Proposed model of delayed c-Met activation. In NSCLC cells, EGFR ligands induce a delayed accumulation of tyrosine phosphorylated c-Met receptors as well as an increase in total c-Met levels. EGFR ligands activate EGFR tyrosine kinase activity, which leads to rapid c-Src signaling and new gene transcription that is required for EGFR-c-Met lateral communication. At delayed time points, activated c-Src continues to accumulate, and c-Met is phosphorylated to maximize EGFR-activated phenotypes of invasion and motility. Production of unidentified proteins might cooperate with c-Src to cause c-Met phosphorylation. This could occur by promoting the clustering of c-Met molecules.
Figure 5
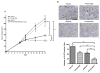
EGFR relies on c-Met for maximal induction of EGFR phenotypes. 201T and A549 cells were serum deprived for 24 h prior to all 48 h phenotypic assays stimulated with EGF (50 ng/mL) or HGF (50 ng/mL). For experiments involving PF2341066 (1 μM), cells were pretreated with the c-Met inhibitor or DMSO for 2 h. For c-Met siRNA transfection experiments, 201T cells were plated and subjected to non-targeting siRNA or c-Met siRNA. Following transfection, cells were serum deprived in 24-well Matrigel invasion chambers or 12-well plates for wound healing assays. (A) Cells were plated in Matrigel invasion chambers with growth factors added to the lower chamber only. Invading cells of three independent experiments were counted. (B) Cells were grown on 12-well plates prior to serum starvation and wounding. Addition of ligands followed c-Met inhibitor pretreatment or siRNA knockdown. Wounds were imaged at 0 and 48 h by 10X light microscopy. Migrating cells were measured by comparing 48 h wound size to initial wound size and expressed as percent wound closure. Mean of three independent samples per treatment group. (C) Invasion and wound healing assays were repeated in 201T cells with c-Met or non-targeting siRNA knockdown as described previously. Mean of three independent samples per treatment group. (D) 201T cells were mock transfected or treated with either non-targeting siRNA (NT) or c-Met siRNA for 8 h. After 48 h, cell lysates were prepared and analyzed for total c-Met levels, c-Src, and β-Actin. (E) 201T cells were serum-deprived for 2 days prior to pretreatment with PF2341066 (1 μM) followed by stimulation with either EGF (10 nM) or HGF (10 ng/mL). In separate experiments, cell lysates were analyzed for MAPK and Akt phosphorylation and expression. ***, P < 0.0005; **, P < 0.005; *, P < 0.05 Student’s t test.
Figure 6
Simultaneous targeting of EGFR and c-Met causes enhanced 201T xenograft tumor growth inhibition. (A) 201T cells (2×106) were injected into nude mice on day 0. On day 10, tumors were measured and experimental treatments were initiated. PF2341066 (50 mg/kg), gefitinib (150 mg/kg), and vehicle control (0.9% saline/1% Tween-80), were administered daily by oral gavage until day 28. (B) Representative Ki-67 stained sections of xenograft tumor imaged at 20X magnification. Quantitation of Ki-67 staining was performed by counting 5 fields at 40X magnification. n = 5; ***, P < 0.0005; **, P < 0.005; *, P < 0.05 Student’s t test with Welch correction. Everything is compared to the vehicle except where indicated.
Similar articles
- Met and c-Src cooperate to compensate for loss of epidermal growth factor receptor kinase activity in breast cancer cells.
Mueller KL, Hunter LA, Ethier SP, Boerner JL. Mueller KL, et al. Cancer Res. 2008 May 1;68(9):3314-22. doi: 10.1158/0008-5472.CAN-08-0132. Cancer Res. 2008. PMID: 18451158 Free PMC article. - Combining onartuzumab with erlotinib inhibits growth of non-small cell lung cancer with activating EGFR mutations and HGF overexpression.
Sano Y, Hashimoto E, Nakatani N, Abe M, Satoh Y, Sakata K, Fujii T, Fujimoto-Ouchi K, Sugimoto M, Nagahashi S, Aoki M, Motegi H, Sasaki E, Yatabe Y. Sano Y, et al. Mol Cancer Ther. 2015 Feb;14(2):533-41. doi: 10.1158/1535-7163.MCT-14-0456. Epub 2014 Dec 18. Mol Cancer Ther. 2015. PMID: 25522765 - Combined therapy with mutant-selective EGFR inhibitor and Met kinase inhibitor for overcoming erlotinib resistance in EGFR-mutant lung cancer.
Nakagawa T, Takeuchi S, Yamada T, Nanjo S, Ishikawa D, Sano T, Kita K, Nakamura T, Matsumoto K, Suda K, Mitsudomi T, Sekido Y, Uenaka T, Yano S. Nakagawa T, et al. Mol Cancer Ther. 2012 Oct;11(10):2149-57. doi: 10.1158/1535-7163.MCT-12-0195. Epub 2012 Jul 25. Mol Cancer Ther. 2012. PMID: 22844075 - [Research progress of HGF/MET signaling pathway in EGFR-TKI resistance in non-small cell lung cancer].
Song S, Bi M. Song S, et al. Zhongguo Fei Ai Za Zhi. 2014 Oct 20;17(10):755-9. doi: 10.3779/j.issn.1009-3419.2014.10.08. Zhongguo Fei Ai Za Zhi. 2014. PMID: 25342043 Free PMC article. Review. Chinese. - Targeting the MET gene for the treatment of non-small-cell lung cancer.
Gelsomino F, Facchinetti F, Haspinger ER, Garassino MC, Trusolino L, De Braud F, Tiseo M. Gelsomino F, et al. Crit Rev Oncol Hematol. 2014 Feb;89(2):284-99. doi: 10.1016/j.critrevonc.2013.11.006. Epub 2013 Dec 1. Crit Rev Oncol Hematol. 2014. PMID: 24355409 Review.
Cited by
- Synthesis and biological evaluation of novel (E)-_N'_-benzylidene hydrazides as novel c-Met inhibitors through fragment based virtual screening.
Liang JW, Li SL, Wang S, Li WQ, Meng FH. Liang JW, et al. J Enzyme Inhib Med Chem. 2020 Dec;35(1):468-477. doi: 10.1080/14756366.2019.1702655. J Enzyme Inhib Med Chem. 2020. PMID: 31902266 Free PMC article. - Suppression of tumor invasion and metastasis by concurrent inhibition of c-Met and VEGF signaling in pancreatic neuroendocrine tumors.
Sennino B, Ishiguro-Oonuma T, Wei Y, Naylor RM, Williamson CW, Bhagwandin V, Tabruyn SP, You WK, Chapman HA, Christensen JG, Aftab DT, McDonald DM. Sennino B, et al. Cancer Discov. 2012 Mar;2(3):270-87. doi: 10.1158/2159-8290.CD-11-0240. Epub 2012 Feb 24. Cancer Discov. 2012. PMID: 22585997 Free PMC article. - Met kinetic signature derived from the response to HGF/SF in a cellular model predicts breast cancer patient survival.
Stein GY, Yosef N, Reichman H, Horev J, Laser-Azogui A, Berens A, Resau J, Ruppin E, Sharan R, Tsarfaty I. Stein GY, et al. PLoS One. 2012;7(9):e45969. doi: 10.1371/journal.pone.0045969. Epub 2012 Sep 25. PLoS One. 2012. PMID: 23049908 Free PMC article. - First-in-Human Phase I Study of Merestinib, an Oral Multikinase Inhibitor, in Patients with Advanced Cancer.
He AR, Cohen RB, Denlinger CS, Sama A, Birnbaum A, Hwang J, Sato T, Lewis N, Mynderse M, Niland M, Giles J, Wallin J, Moser B, Zhang W, Walgren R, Plimack ER. He AR, et al. Oncologist. 2019 Sep;24(9):e930-e942. doi: 10.1634/theoncologist.2018-0411. Epub 2019 Mar 4. Oncologist. 2019. PMID: 30833489 Free PMC article. Clinical Trial. - Novel strategy for a bispecific antibody: induction of dual target internalization and degradation.
Lee JM, Lee SH, Hwang JW, Oh SJ, Kim B, Jung S, Shim SH, Lin PW, Lee SB, Cho MY, Koh YJ, Kim SY, Ahn S, Lee J, Kim KM, Cheong KH, Choi J, Kim KA. Lee JM, et al. Oncogene. 2016 Aug 25;35(34):4437-46. doi: 10.1038/onc.2015.514. Epub 2016 Feb 8. Oncogene. 2016. PMID: 26853467
References
- Bergstrom JD, Westermark B, Heldin NE. Exp Cell Res. 2000;259:293–9. - PubMed
- Bottaro DP, Rubin JS, Faletto DL, Chan AM, Kmiecik TE, Vande Woude GF, Aaronson SA. Science. 1991;251:802–4. - PubMed
- Danilkovitch-Miagkova A, Angeloni D, Skeel A, Donley S, Lerman M, Leonard EJ. J Biol Chem. 2000;275:14783–6. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- R01 CA079882-12/CA/NCI NIH HHS/United States
- R01 CA079882-10A1/CA/NCI NIH HHS/United States
- R01 CA79882/CA/NCI NIH HHS/United States
- R01 CA079882-11/CA/NCI NIH HHS/United States
- R01 CA079882/CA/NCI NIH HHS/United States
- R01 CA079882-13/CA/NCI NIH HHS/United States
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
Research Materials
Miscellaneous