CD40 agonists alter tumor stroma and show efficacy against pancreatic carcinoma in mice and humans - PubMed (original) (raw)
Clinical Trial
. 2011 Mar 25;331(6024):1612-6.
doi: 10.1126/science.1198443.
Elena G Chiorean, Matthew P Fishman, Babak Saboury, Ursina R Teitelbaum, Weijing Sun, Richard D Huhn, Wenru Song, Dongguang Li, Leslie L Sharp, Drew A Torigian, Peter J O'Dwyer, Robert H Vonderheide
Affiliations
- PMID: 21436454
- PMCID: PMC3406187
- DOI: 10.1126/science.1198443
Clinical Trial
CD40 agonists alter tumor stroma and show efficacy against pancreatic carcinoma in mice and humans
Gregory L Beatty et al. Science. 2011.
Abstract
Immunosuppressive tumor microenvironments can restrain antitumor immunity, particularly in pancreatic ductal adenocarcinoma (PDA). Because CD40 activation can reverse immune suppression and drive antitumor T cell responses, we tested the combination of an agonist CD40 antibody with gemcitabine chemotherapy in a small cohort of patients with surgically incurable PDA and observed tumor regressions in some patients. We reproduced this treatment effect in a genetically engineered mouse model of PDA and found unexpectedly that tumor regression required macrophages but not T cells or gemcitabine. CD40-activated macrophages rapidly infiltrated tumors, became tumoricidal, and facilitated the depletion of tumor stroma. Thus, cancer immune surveillance does not necessarily depend on therapy-induced T cells; rather, our findings demonstrate a CD40-dependent mechanism for targeting tumor stroma in the treatment of cancer.
Trial registration: ClinicalTrials.gov NCT00711191.
Figures
Fig. 1
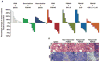
Agonist CD40 mAb in combination with gemcitabine induces clinical responses in patients with surgically incurable PDA. (A) Best overall percentage of change from baseline in tumor target lesion measurement shown as a waterfall plot. *Patient 10061001 was defined as PD because of the appearance of a new nontarget lesion. **Patient 10031001 did not obtain posttherapy scans because of clinical deterioration from disease progression after one dose of CP-870,893. ***Patient 10031010 came off the study after one dose of CP-870,893 but restarted gemcitabine alone and achieved a PR. (B) CT imaging obtained at baseline and end of cycle 3. The primary pancreatic tumor and two metastatic liver lesions are marked by arrows, with the longest dimension annotated. (C) Histopathology of a biopsied metastatic lesion [from patient 10031016 (left)] and a surgically resected primary pancreatic lesion [from patient 10061003 (right)]. Both patients achieved a PR. Patient 10031016 underwent tumor biopsy after completing four cycles of therapy; patient 10061003 underwent surgical resection of the primary tumor after 12 cycles of therapy. Arrows (left) indicate a macrophage-dominated inflammatory infiltrate within extensive tumor necrosis. Arrows (right) identify polymorphonuclear infiltrating cells without lymphocytes; arrowheads mark tumor cells. Scale bars, 50 μm.
Fig. 2
Antitumor activity of agonist CD40 mAb in KPC mice is T cell–independent. (A) KPC mice were treated with gemcitabine or phosphate-buffered saline (PBS) on day 0 and day 7, with control IgG2a or FGK45 administered on day 2. Cohorts of KPC mice receiving treatment with FGK45 were also depleted of CD4+ or CD8+ cells or both CD4+ and CD8+ cells with the use of GK1.5 and 2.43 antibodies, respectively, on days −1, 0, 1, 3, 7, and 10. Percent change in tumor volume from day −1 (baseline) to day +14 is shown for each mouse as a waterfall plot (in comparison with PBS + IgG2a, gemcitabine + FGK45: P < 0.05; gemcitabine + IgG2a: P = 1.00; PBS + FGK45: P < 0.05; FGK45 + GK1.5: P < 0.05; FGK + 2.43: P < 0.05; FGK45 + GK1.5 + 2.43: P < 0.05; Fisher’s exact test). Hematoxylin and eosin (H&E) histology [(B) to (E)] and CD3 immunohistochemistry [(F) to (I)] are shown for tumors from KPC mice treated with IgG2a (B and F) or FGK45 (C to E and G to I). Responders (D), (E), (H), and (I) were defined as FGK45-treated mice that demonstrated tumor regression by ultrasound analysis. FGK45-treated mice with tumor progression by ultrasound were defined as nonresponders (C) and (G). Scale bars, 50 μm.
Fig. 3
Agonist CD40 mAb targets systemic macrophages before their infiltration of tumor. Immunohistochemistry was used to quantify leukocyte infiltration (A) into peripancreatic lymph nodes (A, B, and F) and KPC tumor (A, C, D, E, and G) by staining with a biotinylated goat antibody directed against rat IgG to detect FGK45 or RB6-8C5 bound to cells at 10 min (A) to (D) or 18 hours (A) and (E) to (G) after treatment. Macrophages were depleted with CELs before FGK45 treatment (A) and (G). LN, peripancreatic lymph node; T, tumor; scale bars, 50 μm. Error bars in (A) represent SD. (H) A heat map displaying the flow cytometric analysis of tumor-associated macrophages from individual KPC mice (columns) for expression of cell surface molecules (rows) 3 days after treatment with control IgG2a compared with FGK45. (Top) The percentage of tumor-associated macrophages with surface molecule expression. (Bottom) Mean fluorescence intensity as the number of standard deviations from the mean, which was determined from treatment with control IgG2a. P values are based on Student’s t test; nd, not determined.
Fig. 4
CD40 activated tumor-infiltrating macrophages mediate tumor regression. (A) KPC mice were treated with control IgG2a or FGK45 or FGK45 plus depletion of systemic macrophages by means of CELs. Shown is a waterfall plot displaying percent change in tumor volume from baseline to day +14 (in comparison with IgG2a, FGK45: P < 0.05; FGK45 + CELs: P = 1.00; Fisher’s exact test). (B) F4/80+ tumor-associated macrophages were isolated from KPC mice that had been treated with FGK45 (blue squares) or control IgG2a (green circles) and incubated with KPC-derived tumor cell lines. Tumor cell death in vitro was measured by 7-aminoactinomycin D (7-AAD) labeling and flow cytometric analysis at 24 hours. Shown is a representative assay from two independent experiments each performed in triplicate. Means ± SD are depicted; *P < 0.05, Student’s t test. (C) Cleaved caspase 3 expression on KPC tumors was determined by immunohistochemistry 18 hours after treatment with control IgG2a (top panel) or FGK45 (bottom panel). (D) to (L) KPC tumors were analyzed 18 hours after treatment with control IgG2a (D, F, and H), FGK45 (E, G, and I), or FGK45 + CEL (J, K, and L). Shown are hematoxylin-and-eosin histology (D, E, J); Masson’s trichrome stain to reveal extracellular matrix in blue [(F), (G), and (K)], and immunohistochemistry for collagen I [(H), (I), and (L)]. Scale bars, 50 μm.
Comment in
- Cancer: CD40 agonists--a promising new treatment for pancreatic cancer?
Nanda S. Nanda S. Nat Rev Gastroenterol Hepatol. 2011 Jun;8(6):300. doi: 10.1038/nrgastro.2011.65. Nat Rev Gastroenterol Hepatol. 2011. PMID: 21643028 No abstract available.
Similar articles
- A phase I study of an agonist CD40 monoclonal antibody (CP-870,893) in combination with gemcitabine in patients with advanced pancreatic ductal adenocarcinoma.
Beatty GL, Torigian DA, Chiorean EG, Saboury B, Brothers A, Alavi A, Troxel AB, Sun W, Teitelbaum UR, Vonderheide RH, O'Dwyer PJ. Beatty GL, et al. Clin Cancer Res. 2013 Nov 15;19(22):6286-95. doi: 10.1158/1078-0432.CCR-13-1320. Epub 2013 Aug 27. Clin Cancer Res. 2013. PMID: 23983255 Free PMC article. Clinical Trial. - Exclusion of T Cells From Pancreatic Carcinomas in Mice Is Regulated by Ly6C(low) F4/80(+) Extratumoral Macrophages.
Beatty GL, Winograd R, Evans RA, Long KB, Luque SL, Lee JW, Clendenin C, Gladney WL, Knoblock DM, Guirnalda PD, Vonderheide RH. Beatty GL, et al. Gastroenterology. 2015 Jul;149(1):201-10. doi: 10.1053/j.gastro.2015.04.010. Epub 2015 Apr 14. Gastroenterology. 2015. PMID: 25888329 Free PMC article. - Sufficiency of CD40 activation and immune checkpoint blockade for T cell priming and tumor immunity.
Morrison AH, Diamond MS, Hay CA, Byrne KT, Vonderheide RH. Morrison AH, et al. Proc Natl Acad Sci U S A. 2020 Apr 7;117(14):8022-8031. doi: 10.1073/pnas.1918971117. Epub 2020 Mar 25. Proc Natl Acad Sci U S A. 2020. PMID: 32213589 Free PMC article. - Gemcitabine resistance in pancreatic ductal adenocarcinoma.
Binenbaum Y, Na'ara S, Gil Z. Binenbaum Y, et al. Drug Resist Updat. 2015 Nov;23:55-68. doi: 10.1016/j.drup.2015.10.002. Epub 2015 Nov 3. Drug Resist Updat. 2015. PMID: 26690340 Review. - CD40 immunotherapy for pancreatic cancer.
Vonderheide RH, Bajor DL, Winograd R, Evans RA, Bayne LJ, Beatty GL. Vonderheide RH, et al. Cancer Immunol Immunother. 2013 May;62(5):949-54. doi: 10.1007/s00262-013-1427-5. Epub 2013 Apr 16. Cancer Immunol Immunother. 2013. PMID: 23589109 Free PMC article. Review.
Cited by
- Dendritic cell vaccination and CD40-agonist combination therapy licenses T cell-dependent antitumor immunity in a pancreatic carcinoma murine model.
Lau SP, van Montfoort N, Kinderman P, Lukkes M, Klaase L, van Nimwegen M, van Gulijk M, Dumas J, Mustafa DAM, Lievense SLA, Groeneveldt C, Stadhouders R, Li Y, Stubbs A, Marijt KA, Vroman H, van der Burg SH, Aerts J, van Hall T, Dammeijer F, van Eijck CHJ. Lau SP, et al. J Immunother Cancer. 2020 Jul;8(2):e000772. doi: 10.1136/jitc-2020-000772. J Immunother Cancer. 2020. PMID: 32690771 Free PMC article. - Immunotherapy for gastrointestinal malignancies.
Toomey PG, Vohra NA, Ghansah T, Sarnaik AA, Pilon-Thomas SA. Toomey PG, et al. Cancer Control. 2013 Jan;20(1):32-42. doi: 10.1177/107327481302000106. Cancer Control. 2013. PMID: 23302905 Free PMC article. Review. - Adoptive T Cell Therapy for Solid Tumors: Pathway to Personalized Standard of Care.
Qin SS, Melucci AD, Chacon AC, Prieto PA. Qin SS, et al. Cells. 2021 Apr 5;10(4):808. doi: 10.3390/cells10040808. Cells. 2021. PMID: 33916369 Free PMC article. Review. - To respond or not to respond to CD40 agonism: That is the prediction.
Shi X, Dornan D. Shi X, et al. Oncoimmunology. 2012 Jan 1;1(1):83-85. doi: 10.4161/onci.1.1.17827. Oncoimmunology. 2012. PMID: 22720219 Free PMC article. - What are the macrophages and stellate cells doing in pancreatic adenocarcinoma?
Pandol SJ, Edderkaoui M. Pandol SJ, et al. Front Physiol. 2015 May 15;6:125. doi: 10.3389/fphys.2015.00125. eCollection 2015. Front Physiol. 2015. PMID: 26029109 Free PMC article. Review.
References
- Burris HA, 3rd, et al. J Clin Oncol. 1997;15:2403. - PubMed
- Clark CE, et al. Cancer Res. 2007;67:9518. - PubMed
- Schoenberger SP, Toes RE, van der Voort EI, Offringa R, Melief CJ. Nature. 1998;393:480. - PubMed
- Bennett SR, et al. Nature. 1998;393:478. - PubMed
- Ridge JP, Di Rosa F, Matzinger P. Nature. 1998;393:474. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials