A model to predict cardiovascular events in patients with newly diagnosed Wegener's granulomatosis and microscopic polyangiitis - PubMed (original) (raw)
Randomized Controlled Trial
doi: 10.1002/acr.20433.
Andrew Judge, Rajbir Batra, Oliver Flossmann, Lorraine Harper, Peter Höglund, M Kassim Javaid, David Jayne, Chetan Mukhtyar, Kerstin Westman, John C Davis Jr, Gary S Hoffman, W Joseph McCune, Peter A Merkel, E William St Clair, Philip Seo, Robert Spiera, John H Stone, Raashid Luqmani
Affiliations
- PMID: 21452269
- PMCID: PMC4511092
- DOI: 10.1002/acr.20433
Randomized Controlled Trial
A model to predict cardiovascular events in patients with newly diagnosed Wegener's granulomatosis and microscopic polyangiitis
Ravi Suppiah et al. Arthritis Care Res (Hoboken). 2011 Apr.
Abstract
Objective: To create a prognostic tool to quantify the 5-year cardiovascular (CV) risk in patients with newly diagnosed Wegener's granulomatosis (WG) and microscopic polyangiitis (MPA) without premorbid CV disease.
Methods: We reviewed CV outcomes during the long-term followup of patients in the first 4 European Vasculitis Study Group (EUVAS) trials of WG and MPA. CV events were defined as CV death, stroke, myocardial infarction, coronary artery bypass graft, or percutaneous coronary intervention. Logistic regression was performed to create a model to predict the absolute risk of a CV event. The model was tested using the Wegener's Granulomatosis Etanercept Trial (WGET) cohort.
Results: Seventy-four (13.8%) of 535 patients with 5 years of followup from the EUVAS trials had at least 1 CV event: 33 (11.7%) of 281 WG versus 41 (16.1%) of 254 MPA. The independent determinants of CV outcomes were older age (odds ratio [OR] 1.45, 95% confidence interval [95% CI] 1.11-1.90), diastolic hypertension (OR 1.97, 95% CI 0.98-3.95), and positive proteinase 3 (PR3) antineutrophil cytoplasmic antibody (ANCA) status (OR 0.39, 95% CI 0.20-0.74). The model was validated using the WGET cohort (area under the receiver operating characteristic curve of 0.80).
Conclusion: Within 5 years of diagnosis of WG or MPA, 14% of patients will have a CV event. We have constructed and validated a tool to quantify the risk of a CV event based on age, diastolic hypertension, and PR3 ANCA status in patients without prior CV disease. In patients with vasculitis, PR3 ANCA is associated with a reduced CV risk compared to myeloperoxidase ANCA or negative ANCA status.
Copyright © 2011 by the American College of Rheumatology.
Conflict of interest statement
Competing interests: none
Figures
Figure 1
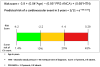
Final model to predict the risk of a cardiovascular event in the first 5 years from diagnosis with microscopic polyangiitis or Wegener’s granulomatosis Five year CV risk is shown as: green = low risk (<10%), orange = moderate risk (10–20%), Red = high risk (>20%).
Figure 2
Receiver operating characteristic curves comparing our new disease specific model with the traditional points based Cox-Framingham model
Figure 3
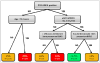
Conditional inference tree showing approximate risk of a cardiovascular event within 5 years based on baseline clinical features % shown is the risk of a cardiovascular event within 5 years of diagnosis of Wegener’s granulomatosis or microscopic polyangiitis. N=Cardiovascular events/number of individuals in Group
Similar articles
- Myeloperoxidase-Antineutrophil Cytoplasmic Antibody (ANCA)-Positive and ANCA-Negative Patients With Granulomatosis With Polyangiitis (Wegener's): Distinct Patient Subsets.
Miloslavsky EM, Lu N, Unizony S, Choi HK, Merkel PA, Seo P, Spiera R, Langford CA, Hoffman GS, Kallenberg CG, St Clair EW, Tchao NK, Fervenza F, Monach PA, Specks U, Stone JH. Miloslavsky EM, et al. Arthritis Rheumatol. 2016 Dec;68(12):2945-2952. doi: 10.1002/art.39812. Arthritis Rheumatol. 2016. PMID: 27428559 Free PMC article. - Uncommon presentations of primary systemic necrotizing vasculitides: the Great Masquerades.
Sharma A, Gopalakrishan D, Nada R, Kumar S, Dogra S, Aggarwal MM, Gupta R, Minz RW, Kakkar N, Vashishtha RK, Singh S. Sharma A, et al. Int J Rheum Dis. 2014 Jun;17(5):562-72. doi: 10.1111/1756-185X.12223. Epub 2013 Nov 14. Int J Rheum Dis. 2014. PMID: 24237487 Review. - [New aspects of antineutrophil cytoplasmic antibody (ANCA) diagnosis in vasculitis].
Csernok E, Reichel P, Gross WL. Csernok E, et al. Z Rheumatol. 2002 Aug;61(4):367-77. doi: 10.1007/s00393-002-0427-1. Z Rheumatol. 2002. PMID: 12426842 Review. German.
Cited by
- Antineutrophil Cytoplasmic Antibody Vasculitis after Coronary Artery Bypass Grafting.
Harris DD, Sabe SA, Ehsan A. Harris DD, et al. Case Rep Surg. 2024 Aug 12;2024:1212538. doi: 10.1155/2024/1212538. eCollection 2024. Case Rep Surg. 2024. PMID: 39161946 Free PMC article. - Stroke frequency, associated factors, and clinical features in primary systemic vasculitis: a multicentric observational study.
Geraldes R, Santos M, Ponte C, Craven A, Barra L, Robson JC, Hammam N, Springer J, Henes J, Hocevar A, Putaala J, Santos E, Rajasekhar L, Daikeler T, Karadag O, Costa A, Khalidi N, Pagnoux C, Canhão P, Melo TPE, Fonseca AC, Ferro JM, Fonseca JE, Suppiah R, Watts RA, Grayson P, Merkel PA, Luqmani RA; DCVAS Study Group. Geraldes R, et al. J Neurol. 2024 Jun;271(6):3309-3320. doi: 10.1007/s00415-024-12251-1. Epub 2024 Mar 12. J Neurol. 2024. PMID: 38472397 Free PMC article. - Risk prediction model for mortality in microscopic polyangiitis: multicentre REVEAL cohort study.
Kotani T, Matsuda S, Okazaki A, Nishioka D, Watanabe R, Gon T, Manabe A, Shoji M, Kadoba K, Hiwa R, Yamamoto W, Hashimoto M, Takeuchi T. Kotani T, et al. Arthritis Res Ther. 2023 Nov 20;25(1):223. doi: 10.1186/s13075-023-03210-8. Arthritis Res Ther. 2023. PMID: 37986108 Free PMC article. - The HAVEN study-hydroxychloroquine in ANCA vasculitis evaluation-a multicentre, randomised, double-blind, placebo-controlled trial: study protocol and statistical analysis plan.
Learoyd AE, Arnold L, Reid F, Beckley-Hoelscher N, Casian A, Sangle S, Morton N, Nel L, Cape A, John S, Kim S, Shivapatham D, Luqmani R, Jayne D, Galloway J, Douiri A, D'Cruz D; HAVEN study group. Learoyd AE, et al. Trials. 2023 Apr 6;24(1):261. doi: 10.1186/s13063-023-07108-3. Trials. 2023. PMID: 37024906 Free PMC article. - Predictors of Acute Myocardial Infraction in Patients With Vasculitis: A Nationwide Inpatient Cross-Sectional Study.
Kaur G, Bajwa AS, Loh CC, Kommuru S, Younis H, Ibrahim Y, Aziz SN, Patel V. Kaur G, et al. Cureus. 2022 Aug 7;14(8):e27751. doi: 10.7759/cureus.27751. eCollection 2022 Aug. Cureus. 2022. PMID: 36106307 Free PMC article.
References
- Mahr AD. Epidemiological features of Wegener’s granulomatosis and microscopic polyangiitis: two diseases or one ‘anti-neutrophil cytoplasm antibodies-associated vasculitis’ entity? APMIS Suppl. 2009:41–7. - PubMed
- Godman GC, Churg J. Wegener’s granulomatosis: pathology and review of the literature. AMA Arch Pathol. 1954;58:533–53. - PubMed
- Hollander D, Manning RT. The use of alkylating agents in the treatment of Wegener’s granulomatosis. Ann Intern Med. 1967;67:393–8. - PubMed
- Jayne D, Rasmussen N, Andrassy K, et al. A randomized trial of maintenance therapy for vasculitis associated with antineutrophil cytoplasmic autoantibodies. N Engl J Med. 2003;349:36–44. - PubMed
- Langford CA, Talar-Williams C, Barron KS, Sneller MC. Use of a cyclophosphamide-induction methotrexate-maintenance regimen for the treatment of Wegener’s granulomatosis: extended follow-up and rate of relapse. Am J Med. 2003;114:463–9. - PubMed
Publication types
MeSH terms
Grants and funding
- M01-RRO-2719/PHS HHS/United States
- K24-AR049185-01/AR/NIAMS NIH HHS/United States
- U01 AR051874/AR/NIAMS NIH HHS/United States
- N01AR92240/AR/NIAMS NIH HHS/United States
- U54 RR019497/RR/NCRR NIH HHS/United States
- FD-R-001652/FD/FDA HHS/United States
- M01 RR001066/RR/NCRR NIH HHS/United States
- U54-AR057319/AR/NIAMS NIH HHS/United States
- MO1-RR-30/RR/NCRR NIH HHS/United States
- U54 RR019497-01/RR/NCRR NIH HHS/United States
- K24 AR002126-01/AR/NIAMS NIH HHS/United States
- U01-AR1874/AR/NIAMS NIH HHS/United States
- 1-U54-RR01949/RR/NCRR NIH HHS/United States
- U54 AR057319-06/AR/NIAMS NIH HHS/United States
- K24-AR02126-04/AR/NIAMS NIH HHS/United States
- K24 AR049185-01/AR/NIAMS NIH HHS/United States
- M01 RR000533-31/RR/NCRR NIH HHS/United States
- K24 AR002224/AR/NIAMS NIH HHS/United States
- M01-RRO-00533/PHS HHS/United States
- N01-AR92240)/AR/NIAMS NIH HHS/United States
- K24-AR2224-01A1/AR/NIAMS NIH HHS/United States
- U01 AR051874-03/AR/NIAMS NIH HHS/United States
- K24 AR049185/AR/NIAMS NIH HHS/United States
- K24 AR002224-03/AR/NIAMS NIH HHS/United States
- U54 AR057319/AR/NIAMS NIH HHS/United States
- M01-RRO-0042/PHS HHS/United States
LinkOut - more resources
Full Text Sources
Medical
Research Materials