The CD40 agonist antibody CP-870,893 enhances dendritic cell and B-cell activity and promotes anti-tumor efficacy in SCID-hu mice - PubMed (original) (raw)
The CD40 agonist antibody CP-870,893 enhances dendritic cell and B-cell activity and promotes anti-tumor efficacy in SCID-hu mice
Ronald P Gladue et al. Cancer Immunol Immunother. 2011 Jul.
Abstract
CD40 is a member of the TNF family of receptors that has been shown to play a crucial role in enhancing dendritic cell activity and fostering anti-tumor immune responses. In this study, we demonstrate the in vitro properties and in vivo efficacious activity of the CD40 agonist antibody, CP-870,893. CP-870,893 is a fully human, IgG2 antibody that selectively interacts with CD40 at a site distinct from its ligand-binding region with a KD of 0.4 nM. It enhances the expression of MHC class II, CD54, CD86, and CD23 on human B cells in vitro. CP-870,893 also enhances dendritic cell activity as evidenced by cytokine secretion (IL-12, IL-23, IL-8), the upregulation of CD86 and CD83, and the ability to prime T cells to secrete IFNγ. In SCID-beige mice, a single parenteral injection of CP-870,893 was therapeutically effective against several CD40(pos) human tumors (B-cell lymphoma, breast, colon, and prostate) indicating direct effects on tumor cell survival and/or growth. When mice were co-implanted with human T cells and dendritic cells, the activity of CP-870,893 against CD40(pos) tumors increased, and efficacy was also observed against CD40(neg) and CD40(low) tumors demonstrating the ability of CP-870,893 to enhance anti-tumor immune function in vivo. These studies suggest that CP-870,893 has the potential to be efficacious against a wide range of tumor types through both direct and immune-mediated effects.
Figures
Fig. 1
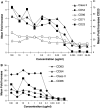
Effects of CP-870,893 on cell surface marker upregulation on human CD19+ B cells and human mDC. a CP-870,893 or anti-KLH control was added to heparinized human whole blood for 24 h, and cell surface upregulation of various markers was determined using flow cytometry by gating on CD19+ B cells. Each data point is the mean of >6 experiments using different human donors. b mDCs were prepared as described in the materials and methods and incubated with various concentrations of CP-870,893 or an anti-KLH control antibody for 24 h. The upregulation of cell surface markers was determined by flow cytometry. Each data point is the mean of at least 6 studies
Fig. 2
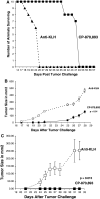
CP-870,893 prevents mortality induced by several _B_-cell lymphomas. a CP-870,893 was administered i.p. followed by the injection of 5 × 106 Daudi cells (IV). Mortality was monitored for 50 days. The MST was 25 days for the anti-KLH group and 48 days for the animals treated with CP-870,893. The group size was 10 animals per group and the study is representative of 5 separate studies. b CP-870,893 was administered i.p. at the time of s.c. tumor challenge with the _B_-cell lymphoma Daudi. Tumor size is represented in mm2. Each data point is the mean ± the SE for 5 animals per group and is representative of 3 separate experiments. c CP-870,893 was administered i.p. at the time of s.c. tumor challenge with the _B_-cell lymphoma Jijoye. Each data point refers to the mean tumor size in mm2 ± the SE for 5 animals per group. The study is representative of 3 separate studies
Fig. 3
Effect of human T cells and mDCs on the in vivo activity of CP-870,893 in SCID-beige mice. a Dose-dependant inhibition of the CD40pos tumor Raji by CP-870,893 in the presence or absence of human T cells and mDC. CP-870,893 was administered i.p. at the time of s.c. tumor challenge. The data points represent the tumor size (mm2) for each individual animal taken on day 21 after tumor challenge. The mean tumor size for each treatment group (N = 10) is indicated by the horizontal line. The study is representative of at least 3 separate experiments. Significance is indicated as compared to the anti-KLH control. Significance in comparing groups with and without T cells and mDC are as follows: 0.1 mg/kg group P < 0.01; 0.01 mg/kg group P < 0.01; 0.001 mg/kg group P = 0.0103; 0.0001 mg/kg group P = 0.26. b Dose-dependant inhibition of the CD40neg tumor K562 by CP-870,893 in the presence human T cells and mDC. Animals received a single injection (IP) of various dose levels of CP-870,893 or 10 mg/kg anti-KLH at the time of s.c. tumor challenge. The data are representative of 3 experiments
Fig. 4
CP-870,893 prevents the growth of the human breast tumor cell line, BT 474 in the presence of human T cells and mDCs. BT474 tumor cells were administered s.c. along with human T cells and mDCs. a CP-870,893 was administered i.p. at the time of tumor challenge. Each data point refers to the tumor size of an individual animal measured on day 53 after challenge, a time when control animals reach a tumor size where they were euthanized. The horizontal lines represent the mean tumor size. b CP-870,893 was administered at a dose of 1.0 mg/kg once tumors reached a size of 100 mm2. Each data point refers to the tumor size of an individual animal measured on day 52 after challenge, a time when control animals reach a tumor size where they were euthanized. The horizontal lines represent the mean tumor size for all animals in that group
Similar articles
- Activation of human B cells by the agonist CD40 antibody CP-870,893 and augmentation with simultaneous toll-like receptor 9 stimulation.
Carpenter EL, Mick R, Rüter J, Vonderheide RH. Carpenter EL, et al. J Transl Med. 2009 Nov 11;7:93. doi: 10.1186/1479-5876-7-93. J Transl Med. 2009. PMID: 19906293 Free PMC article. - The human anti-CD40 agonist antibody mitazalimab (ADC-1013; JNJ-64457107) activates antigen-presenting cells, improves expansion of antigen-specific T cells, and enhances anti-tumor efficacy of a model cancer vaccine in vivo.
Deronic A, Nilsson A, Thagesson M, Werchau D, Enell Smith K, Ellmark P. Deronic A, et al. Cancer Immunol Immunother. 2021 Dec;70(12):3629-3642. doi: 10.1007/s00262-021-02932-5. Epub 2021 May 5. Cancer Immunol Immunother. 2021. PMID: 33948686 Free PMC article. - Distinctive maturation of in vitro versus in vivo anti-CD40 mAb-matured dendritic cells in mice.
Frleta D, Lin JT, Quezada SA, Wade TK, Barth RJ, Noelle RJ, Wade WF. Frleta D, et al. J Immunother. 2003 Jan-Feb;26(1):72-84. doi: 10.1097/00002371-200301000-00008. J Immunother. 2003. PMID: 12514431 - Mechanistic basis of co-stimulatory CD40-CD40L ligation mediated regulation of immune responses in cancer and autoimmune disorders.
Chand Dakal T, Dhabhai B, Agarwal D, Gupta R, Nagda G, Meena AR, Dhakar R, Menon A, Mathur R, Mona, Yadav V, Sharma A. Chand Dakal T, et al. Immunobiology. 2020 Mar;225(2):151899. doi: 10.1016/j.imbio.2019.151899. Epub 2019 Dec 17. Immunobiology. 2020. PMID: 31899051 Review. - Functions of CD40 on B cells, dendritic cells and other cells.
van Kooten C, Banchereau J. van Kooten C, et al. Curr Opin Immunol. 1997 Jun;9(3):330-7. doi: 10.1016/s0952-7915(97)80078-7. Curr Opin Immunol. 1997. PMID: 9203418 Review.
Cited by
- Expression of CD40 and growth-inhibitory activity of CD40 agonist in ovarian carcinoma cells.
Zhou Y, He J, Gou LT, Mu B, Liao WC, Ma C, Tang P, Zhou SJ, Zhou YJ, Yang JL. Zhou Y, et al. Cancer Immunol Immunother. 2012 Oct;61(10):1735-43. doi: 10.1007/s00262-011-1194-0. Epub 2012 Mar 11. Cancer Immunol Immunother. 2012. PMID: 22406982 Free PMC article. - Mitigating the toxic effects of anticancer immunotherapy.
Gangadhar TC, Vonderheide RH. Gangadhar TC, et al. Nat Rev Clin Oncol. 2014 Feb;11(2):91-9. doi: 10.1038/nrclinonc.2013.245. Epub 2014 Jan 21. Nat Rev Clin Oncol. 2014. PMID: 24445516 Review. - Human Anti-CD40 Antibody and Poly IC:LC Adjuvant Combination Induces Potent T Cell Responses in the Lung of Nonhuman Primates.
Thompson EA, Liang F, Lindgren G, Sandgren KJ, Quinn KM, Darrah PA, Koup RA, Seder RA, Kedl RM, Loré K. Thompson EA, et al. J Immunol. 2015 Aug 1;195(3):1015-24. doi: 10.4049/jimmunol.1500078. Epub 2015 Jun 29. J Immunol. 2015. PMID: 26123354 Free PMC article. - Characteristics and clinical trial results of agonistic anti-CD40 antibodies in the treatment of malignancies.
Li DK, Wang W. Li DK, et al. Oncol Lett. 2020 Nov;20(5):176. doi: 10.3892/ol.2020.12037. Epub 2020 Aug 31. Oncol Lett. 2020. PMID: 32934743 Free PMC article. Review. - Therapeutic targeting of tumour myeloid cells.
Barry ST, Gabrilovich DI, Sansom OJ, Campbell AD, Morton JP. Barry ST, et al. Nat Rev Cancer. 2023 Apr;23(4):216-237. doi: 10.1038/s41568-022-00546-2. Epub 2023 Feb 6. Nat Rev Cancer. 2023. PMID: 36747021 Review.
References
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous