Defining the origins of Ras/p53-mediated squamous cell carcinoma - PubMed (original) (raw)
Defining the origins of Ras/p53-mediated squamous cell carcinoma
Andrew C White et al. Proc Natl Acad Sci U S A. 2011.
Abstract
The precise identity of cancer cells of origin and the molecular events of tumor initiation in epidermal squamous cell carcinoma (SCC) are unknown. Here we show that malignancy potential is related to the developmental capacity of the initiating cancer cell in a genetically defined, intact, and inducible in vivo model. Specifically, these data demonstrate that SCCs can originate from inside the hair follicle stem cell (SC) niche or from immediate progenitors, whereas more developmentally restricted progeny, the transit amplifying (TA) cells, are unable to generate even benign tumors in the same genetic context. Using a temporal model of tumorigenesis in situ, we highlight the phenotypes of cancer progression from the hair follicle SC niche, including hyperplasia, epithelial to mesenchymal transition, and SCC formation. Furthermore, we provide insights into the inability of hair follicle TA cells to respond to tumorigenic stimuli.
Conflict of interest statement
The authors declare no conflict of interest.
Figures
Fig. 1.
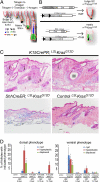
Phenotypes resulting from KrasG12D expression in SC and TA cell populations of the hair follicle. (A) Model depicting the location and promoter specificity of SCs (K15+) and TA cells (Shh+) of the hair follicle during the growing phase of the hair cycle (anagen). In addition, a number of relevant cell types are shown (cp, companion layer; cx, hair cortex; ors, outer root sheath; dp, dermal papillae; mx, matrix; ge, hair germ). (B) Model of the targeting strategy to express oncogenic KrasG12D in either bulge SCs or matrix TA cells. (C) Histopathology of phenotypes induced by KrasG12D expression in the follicular bulge, including hyperplastic sebaceous glands (i), hyperplastic hair follicles (ii), and follicular cysts (iii). No apparent abnormal hair follicle phenotypes were detected in ShhCreER; KrasG12D skin. (Magnification, 10×.) (D) Quantification of phenotypes in treated and untreated mice. The indicated phenotypes were quantified on a per-follicle basis on the indicated genotypes with or without mifepristone treatment. The timecourse indicates the number of weeks after anagen, as measured by regrowth after shaving. The number of phenotypes found in treated animals was significantly increased over those found in untreated animals, even at timepoints far later than those used for phenotypic analysis in treated animals. *P ≤ 0.049).
Fig. 2.
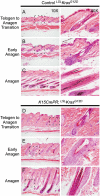
Bulge expansion resulting from KrasG12D expression. (A and D) During telogen, control and _KrasG12D_-induced follicles appear similar. (B and E) At the onset of anagen, before full hair follicle formation but after SC exit from quiescence, bulge expansion is evident in _KrasG12D_-induced animals. (C and F) After formation of a full hair follicle, notable ORS hyperplasia can be detected in most follicles.
Fig. 3.
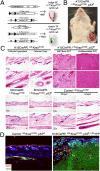
Expression of oncogenic KrasG12D and p53 ablation in the SC niche generates SCC, whereas the same stimulus in TA cells does not. (A) Model depicting the strategy for KrasG12D expression combined with deletion of the tumor suppressor p53. (B) Macroscopic phenotype of an SCC derived from the hair follicle bulge in K15CrePR; KrasG12D; p53ff mice. Tumors can be detected on the animal within 10 wk after mifepristone treatment. (C) K15-CrePR; KrasG12D; p53ff skin demonstrates hyperplastic sebaceous glands, hyperplastic hair follicles, epidermal cysts, transformed spindle cells invading and populating the dermis, and large exophytic SCCs. No skin abnormalities were detected in ShhCreER; KrasG12D; p53ff animals. (D) Cancer cells invading the dermis were derived from YFP-labeled bulge K15CrePR; KrasG12D; p53ff; LSL-YFP cells. Asterisks indicate Krt5+ remnant hair follicles.
Fig. 4.
Activation of signaling pathways downstream of Ras signaling during tumorigenesis arising from the bulge. (A) Erk activity (p-Erk) appeared in hyperplastic follicles but not in follicular cysts or in SCCs. (B) Akt activity (p-Akt) was detected in bulges, in non-ORS cells of hyperplastic follicles, cysts, and in remnant potions of hair follicles within SCCs (Inset). (C) Phosphorylated S6 (p-S6) was found in hyperplastic follicles, cysts and, at a lower level, throughout SCCs. (Magnification, 20×.)
Similar articles
- Identifying the cellular origin of squamous skin tumors.
Lapouge G, Youssef KK, Vokaer B, Achouri Y, Michaux C, Sotiropoulou PA, Blanpain C. Lapouge G, et al. Proc Natl Acad Sci U S A. 2011 May 3;108(18):7431-6. doi: 10.1073/pnas.1012720108. Epub 2011 Apr 18. Proc Natl Acad Sci U S A. 2011. PMID: 21502497 Free PMC article. - Stem cell quiescence acts as a tumour suppressor in squamous tumours.
White AC, Khuu JK, Dang CY, Hu J, Tran KV, Liu A, Gomez S, Zhang Z, Yi R, Scumpia P, Grigorian M, Lowry WE. White AC, et al. Nat Cell Biol. 2014 Jan;16(1):99-107. doi: 10.1038/ncb2889. Epub 2013 Dec 15. Nat Cell Biol. 2014. PMID: 24335650 Free PMC article. - Pten loss in Lgr5+ hair follicle stem cells promotes SCC development.
Chen H, Wang X, Chen Y, Han J, Kong D, Zhu M, Fu X, Wu Y. Chen H, et al. Theranostics. 2019 Oct 22;9(26):8321-8331. doi: 10.7150/thno.35467. eCollection 2019. Theranostics. 2019. PMID: 31754399 Free PMC article. - Hair follicle stem cells: walking the maze.
Tiede S, Kloepper JE, Bodò E, Tiwari S, Kruse C, Paus R. Tiede S, et al. Eur J Cell Biol. 2007 Jul;86(7):355-76. doi: 10.1016/j.ejcb.2007.03.006. Epub 2007 Jun 18. Eur J Cell Biol. 2007. PMID: 17576022 Review. - Targeted Therapy Against the Cell of Origin in Cutaneous Squamous Cell Carcinoma.
Goldie SJ, Chincarini G, Darido C. Goldie SJ, et al. Int J Mol Sci. 2019 May 5;20(9):2201. doi: 10.3390/ijms20092201. Int J Mol Sci. 2019. PMID: 31060263 Free PMC article. Review.
Cited by
- A keratin 15 containing stem cell population from the hair follicle contributes to squamous papilloma development in the mouse.
Li S, Park H, Trempus CS, Gordon D, Liu Y, Cotsarelis G, Morris RJ. Li S, et al. Mol Carcinog. 2013 Oct;52(10):751-9. doi: 10.1002/mc.21896. Epub 2012 Mar 16. Mol Carcinog. 2013. PMID: 22431489 Free PMC article. - Its written all over your face: The molecular and physiological consequences of aging skin.
Lowry WE. Lowry WE. Mech Ageing Dev. 2020 Sep;190:111315. doi: 10.1016/j.mad.2020.111315. Epub 2020 Jul 15. Mech Ageing Dev. 2020. PMID: 32681843 Free PMC article. Review. - Emerging roles of transit-amplifying cells in tissue regeneration and cancer.
Zhang B, Hsu YC. Zhang B, et al. Wiley Interdiscip Rev Dev Biol. 2017 Sep;6(5):10.1002/wdev.282. doi: 10.1002/wdev.282. Epub 2017 Jul 3. Wiley Interdiscip Rev Dev Biol. 2017. PMID: 28670819 Free PMC article. Review. - Distinct phases of human prostate cancer initiation and progression can be driven by different cell-types.
Stoyanova T, Goldstein AS. Stoyanova T, et al. Cancer Cell Microenviron. 2014;1(3):e90. doi: 10.14800/ccm.90. Cancer Cell Microenviron. 2014. PMID: 26005704 Free PMC article. - Epithelial stem cells, wound healing and cancer.
Arwert EN, Hoste E, Watt FM. Arwert EN, et al. Nat Rev Cancer. 2012 Feb 24;12(3):170-80. doi: 10.1038/nrc3217. Nat Rev Cancer. 2012. PMID: 22362215 Review.
References
- Alam M, Ratner D. Cutaneous squamous-cell carcinoma. N Engl J Med. 2001;344:975–983. - PubMed
- van der Schroeff JG, Evers LM, Boot AJ, Bos JL. Ras oncogene mutations in basal cell carcinomas and squamous cell carcinomas of human skin. J Invest Dermatol. 1990;94:423–425. - PubMed
- Spencer JM, Kahn SM, Jiang W, DeLeo VA, Weinstein IB. Activated ras genes occur in human actinic keratoses, premalignant precursors to squamous cell carcinomas. Arch Dermatol. 1995;131:796–800. - PubMed
- Brown K, Strathdee D, Bryson S, Lambie W, Balmain A. The malignant capacity of skin tumours induced by expression of a mutant H-ras transgene depends on the cell type targeted. Curr Biol. 1998;8:516–524. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
Research Materials
Miscellaneous