Palmitic acid acutely stimulates glucose uptake via activation of Akt and ERK1/2 in skeletal muscle cells - PubMed (original) (raw)
Palmitic acid acutely stimulates glucose uptake via activation of Akt and ERK1/2 in skeletal muscle cells
Jing Pu et al. J Lipid Res. 2011 Jul.
Abstract
Chronic exposure to saturated fatty acids can cause insulin resistance. However, the acute effects of fatty acids are not clear and need to be elucidated because plasma fatty acid concentrations fluctuate postprandially. Here, we present the acute effects of palmitate (PA) on skeletal muscle cells and their underlying molecular mechanisms. Immuno-fluorescence results showed that PA rapidly induced GLUT4 translocation and stimulated glucose uptake in rat skeletal muscle cell line L6. Phosphorylation of AMP-activated protein kinase (AMPK), Akt, and extracellular signal-related kinase1/2 (ERK1/2) was enhanced by PA in a time-dependent manner. Cell surface-bound PA was sufficient to stimulate Akt phosphorylation. The inhibitors of PI3 kinase (PI3K), AMPK, Akt, and ERK1/2 could decrease PA-induced glucose uptake, and PI3K inhibitor decreased AMPK, Akt, and ERK1/2 phosphorylation. Weakening AMPK activity reduced phosphorylation of Akt but not ERK1/2, and Akt inhibitor could not affect ERK1/2 activation either. Meanwhile, ERK1/2 inhibitors had no effect on Akt phosphorylation. Taken together, our data suggest that PA-mediated glucose uptake in skeletal muscle cells may be stimulated by the binding of PA to cell surface and followed by PI3K/AMPK/Akt and PI3K/ERK1/2 pathways independently.
Figures
Fig. 1.
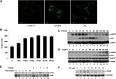
Palmitate stimulates GLUT4 translocation, glucose uptake, and phosphorylation of AMPK, Akt, and ERK. A: L6-GLUT4_myc_ (L6) cells were treated with (right panel) or without (left panel) PA (300 μM) for 30 min or 100 nM insulin (middle panel) for 20 min and washed three times with ice-cold KRBH buffer. Immunofluorescence was performed with anti-myc polyclonal antibody and FITC-conjugated IgG. Fluorescence images were obtained with an Olympus FV500 confocal fluorescence microscope. Bar = 10 μm. B: L6 cells were treated with or without 300 μM PA for the indicated times. Cells were then treated with 3H-labeled 2-deoxy-D-glucose and glucose uptake was assayed. Data are presented as means ± SD (n = 3) from one of four time-independent experiments with triplicate. C, D: L6 myoblasts were incubated in the presence of 300 μM PA for the times indicated (C) or with the indicated concentrations of PA for 30 min (D). Cells were lysed in 2× SDS sample buffer and total protein was subjected to Western blotting using the antibodies indicated. GAPDH was included as an internal control. E: A total of 2 mM palmitate in saline containing 10% FBS was used to perfuse male Sprague-Dawley rats. After perfusion, isolated soleus muscle strips were incubated in 2 mM palmitate at 37°C for the times indicated. Control rats were treated with 10% FBS-containing saline. The cost times for each step are indicated in supplementary Fig. IF. Western blots representing one of three experiments are shown. F: L6 cells were treated as in C for the indicated times and whole cell lysates were subjected to Western blotting.
Fig. 2.
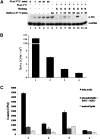
Binding of palmitate to the plasma membrane is required for stimulation of Akt phosphorylation. A: C2C12 mouse muscle cells were treated with 300 μM PA (lanes 2 to 5) for the times indicated or 100 nM insulin (lane 6) for 20 min. By way of comparison, C2C12 cells were incubated in the presence of 300 μM PA at 4°C for 1 h and then washed three times with ice-cold KRBH buffer or 0.4% fatty acid-free BSA solution. After washing, cells were either lysed with 2×SDS sample buffer (lane 7 and lane 13) or incubated in prewarmed KRBH buffer at 37°C for the times indicated (lanes 8 to 11, lane 14). A total of 100 nM insulin in prewarmed KRBH buffer was used to treat cells at 37°C for 20 min (lane 12) as a positive control. After incubation, cells were lysed and total protein was subjected to Western blotting using the antibodies indicated. GAPDH was included as an internal control. K, KRBH; B, BSA; ins, insulin. B: Cells were treated with 300 μM PA plus 0.2 μCi/ml 3H-labeled PA at 4°C for 1 h and washed three times with ice-cold KRBH buffer or 0.4% fatty acid-free BSA solution. Cells were then lysed and radio-labeled lipids were measured by scintillation counting. Data are presented as means ± SD (n = 3). Bar 1: total input. Bar 2: 3H labeled lipids remaining prior to washing. Bar 3: 3H labeled lipids remaining after washing with KRBH. Bar 4: 3H labeled lipids remaining after washing with BSA. Effective binding amount = (Bar 3–Bar 4)/Bar 1 = 0.43% of total input. C: The same experiments were carried out as in B and then cells were incubated at 37°C for 10 min. Then the cells were lysed, and the total lipids were extracted and separated by TLC. 3H-labeled lipids were measured by scintillation counting. Data are presented as means ± SD (n = 3) from one of three time-independent experiments with triplicate. Group 1: lipids from the cells that were washed by KRBH buffer at 4°C. Group 2: lipids from the cells that were washed by BSA solution at 4°C. Group 3: lipids from the cells that were washed by KRBH at 4°C and then incubated at 37°C for 10 min. Group 4: lipids from the cells that were washed by BSA solution at 4°C and then incubated at 37°C for 10 min.
Fig. 3.
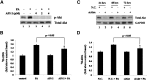
Akt is involved in palmitate-stimulated glucose uptake. A: L6 cells were pretreated with or without API-2 at indicated concentrations for 30 min and then with or without 300 μM PA alone or with inhibitor for 30 min, and total cell lysates were subjected to Western blotting. B: L6 cells were treated with 2 μM API-2 and 300 μM PA as in A and then 3H-labeled 2-deoxy-D-glucose uptake assays were performed. Data are presented as means ± SD (n = 3) from one of three time-independent experiments with triplicate. Differences between PA alone and PA with API-2 were determined using the Student's _t_-test. * P < 0.05. C: L6 cells were nucleofected with siRNA targeting Akt (siAkt) or negative control siRNA (N.C.). The cells were harvested after the indicated transfection times, and the total cell lysates obtained were subjected to Western blotting. D: L6 cells were nucleofected as in C and treated with 300 μM PA for 30 min after 48 h of transfection. 3H-labeled 2-deoxy-D-glucose uptake assays were performed. Data are presented as means ± SD (n = 3) from one of three time-independent experiments with triplicate. Differences in PA effects between N.C.-transfected cells and siAkt-transfected cells were determined using the Student's _t_-test. * P < 0.05.
Fig. 4.
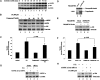
AMPK is involved in palmitate-stimulated glucose uptake by regulating Akt activity. A: L6 cells were treated with 2 mM AICAR for the indicated times, and the total cell lysates obtained were subjected to Western blotting. B: L6 cells were pretreated with or without 10 μM or 20 μM Compound C for 1 h and then treated with 300 μM PA alone or with 10 μM or 20 μM Compound C for 30 min. The total cell lysates were subjected to Western blotting. The images are from different lanes of the same gel. C: L6 cells were pretreated with or without 10 μM Compound C for 1 h and then treated with 300 μM PA alone or with 10 μM Compound C for 30 min. 3H-labeled 2-deoxy-D-glucose uptake assays were performed. Data are presented as means ± SD (n = 3) from one of four time-independent experiments with triplicate. Differences between PA alone and PA with Compound C were determined using the Student's _t_-test. * P < 0.05. D: L6 cells were nucleofected with pcDNA3.1 or AMPK-DN plasmids. After 48 h of transfection, cells were lysed and total proteins were obtained to analyze with anti-myc monoclonal antibody. E: L6 cells were transfected as in D and treated with or without 300 μM PA for 30 min. Cells were lysed in 2× SDS sample buffer, and total protein was subjected to Western blotting using the antibodies indicated. F: L6 cells were transfected and treated as in E and then subjected to 3H-labeled 2-deoxy-D-glucose uptake assays. Data are presented as means ± SD (n = 3) from one of three time-independent experiments with triplicate. Differences in PA effects between mock and AMPK-DN-transfected cells were determined using the Student's _t_-test. * P < 0.05. G: L6 cells were nucleofected with siRNA duplex mixture targeting AMPK α1 and α2 or negative control siRNA (N.C.). The cells were harvested after the indicated transfection times and the total cell lysates obtained were subjected to Western blotting. H: L6 cells were nucleofected as in G and treated with or without 300 μM PA for 30 min after 48 h of transfection. The cells were lysed, and the total proteins obtained were subjected to Western blotting. The images are from different lanes of the same gel.
Fig. 5.
PI3 kinase is involved in palmitate-stimulated glucose uptake, regulating ERK in an AMPK- and Akt-independent manner A: L6 cells were pretreated with or without 50 μM LY294002 for 30 min and then with or without 300 μM PA alone or with inhibitor for 30 min. 3H-labeled 2-deoxy-D-glucose uptake assays were performed. Data are presented as means ± SD (n = 3) from one of three time-independent experiments with triplicate. Differences between PA alone and PA with LY294002 were determined using the Student's _t_-test. * P < 0.05. B: L6 cells were pretreated with or without 50 μM LY294002 for 30 min and then with or without 300 μM PA alone or with inhibitor for 15 min. Cells were lysed and total proteins were subjected to Western blotting. The images are from different lanes of the same gel. C: C2C12 cells were treated with 300 μM PA alone (lane 5) or with 50 μM LY294002 (LY) (lane 6) for 30 min, or 100 nM insulin with or without 50 μM LY294002 for 20 min. After treatment, cells were lysed and total protein was obtained and subjected to Western blotting. The images are from different lanes of the same gel. D: L6 cells were pretreated with or without PD98058 or U0126 at indicated concentrations for 90 min and then treated with 300 μM PA alone or combined with PD98058 or U0126 at the indicated concentrations for 10 min. Cell lysates were obtained and subjected to Western blotting. The images are from different lanes of the same gel. E: L6 cells were treated with or without 10 μM U0126 and 300 μM PA as in B and then subjected to 3H-labeled 2-deoxy-D-glucose uptake assays. Data are presented as means ± SD (n = 3) from one of three time independent experiments with triplicate. Differences in PA effects between control cells and U0126 treated cells were determined using the Student's _t_-test. * P < 0.01. F: L6 cells were pretreated with or without 2 μM API-2 or 50 μM LY294002 for 30 min and then treated with or without 300 μM PA alone or combined with above inhibitors for 10 min. Cell lysates were obtained and subjected to Western blotting. The images are from different lanes of the same gel. G: L6 cells were nucleofected with siRNA duplex mixture targeting AMPK α1 and α2 or negative control siRNA (N.C.) and treated with or without 300 μM PA for the indicated times after 48 h of transfection. The cells were lysed and the total proteins were obtained to subjected to Western blotting.
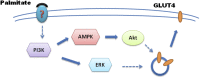
Fig. 6
6. Pathways PI3K/AMPK/Akt and PI3K/ERK1/2 in mediating palmitate-stimulated glucose uptake. In response to plasma membrane-bound palmitate, signal is transduced by activation of PI3K/AMPK/Akt and PI3K/ERK1/2, which in turn leads GLUT4 translocation to plasma membrane and glucose uptake in skeletal muscle cells.
Similar articles
- Diastereomeric mixture of calophyllic acid and isocalophyllic acid stimulates glucose uptake in skeletal muscle cells: involvement of PI-3-kinase- and ERK1/2-dependent pathways.
Prasad J, Maurya CK, Pandey J, Jaiswal N, Madhur G, Srivastava AK, Narender T, Tamrakar AK. Prasad J, et al. Mol Cell Endocrinol. 2013 May 6;370(1-2):11-9. doi: 10.1016/j.mce.2013.02.013. Epub 2013 Feb 19. Mol Cell Endocrinol. 2013. PMID: 23428406 - Karanjin from Pongamia pinnata induces GLUT4 translocation in skeletal muscle cells in a phosphatidylinositol-3-kinase-independent manner.
Jaiswal N, Yadav PP, Maurya R, Srivastava AK, Tamrakar AK. Jaiswal N, et al. Eur J Pharmacol. 2011 Nov 16;670(1):22-8. doi: 10.1016/j.ejphar.2011.08.049. Epub 2011 Sep 14. Eur J Pharmacol. 2011. PMID: 21939653 - Epigallocatechin gallate induces GLUT4 translocation in skeletal muscle through both PI3K- and AMPK-dependent pathways.
Ueda-Wakagi M , Hayashibara K , Nagano T , Ikeda M , Yuan S , Ueda S , Shirai Y , Yoshida KI , Ashida H . Ueda-Wakagi M , et al. Food Funct. 2018 Aug 15;9(8):4223-4233. doi: 10.1039/c8fo00807h. Food Funct. 2018. PMID: 29998274 - Activation of phosphatidylinositol-3 kinase, AMP-activated kinase and Akt substrate-160 kDa by trans-10, cis-12 conjugated linoleic acid mediates skeletal muscle glucose uptake.
Mohankumar SK, Taylor CG, Siemens L, Zahradka P. Mohankumar SK, et al. J Nutr Biochem. 2013 Feb;24(2):445-56. doi: 10.1016/j.jnutbio.2012.01.006. Epub 2012 Jun 15. J Nutr Biochem. 2013. PMID: 22704782 - Chlorogenic acid stimulates glucose transport in skeletal muscle via AMPK activation: a contributor to the beneficial effects of coffee on diabetes.
Ong KW, Hsu A, Tan BK. Ong KW, et al. PLoS One. 2012;7(3):e32718. doi: 10.1371/journal.pone.0032718. Epub 2012 Mar 7. PLoS One. 2012. PMID: 22412912 Free PMC article.
Cited by
- Cardiac metallothionein overexpression rescues diabetic cardiomyopathy in Akt2-knockout mice.
Huang S, Wang J, Men H, Tan Y, Lin Q, Gozal E, Zheng Y, Cai L. Huang S, et al. J Cell Mol Med. 2021 Jul;25(14):6828-6840. doi: 10.1111/jcmm.16687. Epub 2021 May 30. J Cell Mol Med. 2021. PMID: 34053181 Free PMC article. - Targeting the immunoproteasome in hypothalamic neurons as a novel therapeutic strategy for high-fat diet-induced obesity and metabolic dysregulation.
Albornoz N, Álvarez-Indo J, de la Peña A, Arias-Muñoz E, Coca A, Segovia-Miranda F, Kerr B, Budini M, Criollo A, García-Robles MA, Morselli E, Soza A, Burgos PV. Albornoz N, et al. J Neuroinflammation. 2024 Aug 2;21(1):191. doi: 10.1186/s12974-024-03154-z. J Neuroinflammation. 2024. PMID: 39095788 Free PMC article. - Fatty acids increase neuronal hypertrophy of Pten knockdown neurons.
Fricano CJ, Despenza T Jr, Frazel PW, Li M, O'Malley AJ, Westbrook GL, Luikart BW. Fricano CJ, et al. Front Mol Neurosci. 2014 Apr 23;7:30. doi: 10.3389/fnmol.2014.00030. eCollection 2014. Front Mol Neurosci. 2014. PMID: 24795563 Free PMC article. - Dietary protein restriction regulates skeletal muscle fiber metabolic characteristics associated with the FGF21-ERK1/2 pathway.
Li S, Zhong H, Wang Z, Chen J, Huang Z, Zou T, You J. Li S, et al. iScience. 2024 Feb 19;27(3):109249. doi: 10.1016/j.isci.2024.109249. eCollection 2024 Mar 15. iScience. 2024. PMID: 38450157 Free PMC article. - Protective Effects of Hemp (Cannabis sativa) Root Extracts against Insulin-Deficient Diabetes Mellitus In Mice.
Kim Y, Kim W, Kim SH, Sim KS, Kim KH, Cho KH, Kwon GS, Lee JB, Kim JH. Kim Y, et al. Molecules. 2023 Apr 29;28(9):3814. doi: 10.3390/molecules28093814. Molecules. 2023. PMID: 37175224 Free PMC article.
References
- McGarry J. D. 1992. What if Minkowski had been ageusic? An alternative angle on diabetes. Science. 258: 766–835. - PubMed
- DeFronzo R. A., Jacot E., Jequier E., Maeder E., Wahren J., Felber J. P. 1981. The effect of insulin on the disposal of intravenous glucose. Results from indirect calorimetry and hepatic and femoral venous catheterization. Diabetes. 30: 1000–1006. - PubMed
- Shulman G. I., Rothman D. L., Jue T., Stein P., DeFronzo R. A., Shulman R. G. 1990. Quantitation of muscle glycogen synthesis in normal subjects and subjects with non-insulin-dependent diabetes by 13C nuclear magnetic resonance spectroscopy. N. Engl. J. Med. 322: 223–230. - PubMed
- Martin S., Parton R. G. 2006. Lipid droplets: a unified view of a dynamic organelle. Nat. Rev. Mol. Cell Biol. 7: 373–380. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous