The LC3 recruitment mechanism is separate from Atg9L1-dependent membrane formation in the autophagic response against Salmonella - PubMed (original) (raw)
The LC3 recruitment mechanism is separate from Atg9L1-dependent membrane formation in the autophagic response against Salmonella
Shun Kageyama et al. Mol Biol Cell. 2011.
Abstract
Salmonella develops into resident bacteria in epithelial cells, and the autophagic machinery (Atg) is thought to play an important role in this process. In this paper, we show that an autophagosome-like double-membrane structure surrounds the Salmonella still residing within the Salmonella-containing vacuole (SCV). This double membrane is defective in Atg9L1- and FAK family-interacting protein of 200 kDa (FIP200)-deficient cells. Atg9L1 and FIP200 are important for autophagy-specific recruitment of the phosphatidylinositol 3-kinase (PI3K) complex. However, in the absence of Atg9L1, FIP200, and the PI3K complex, LC3 and its E3-like enzyme, the Atg16L complex, are still recruited to Salmonella. We propose that the LC3 system is recruited through a mechanism that is independent of isolation membrane generation.
Figures
FIGURE 1:
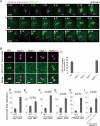
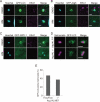
GFP-LC3 association with infected S. typhimurium. (A) MEFs stably expressing GFP-LC3 were infected with S. typhimurium expressing mCherry for 5 min and then washed to remove the inoculum. The cells were imaged at ∼15-s intervals using a fluorescence inverted microscope. Live-cell imaging is shown and the elapsed time after the association of GFP-LC3 is indicated. Scale bar: 1 μm. (B and C) MEFs with deletions in each of the noted Atg genes and their parental cells stably expressing GFP-LC3 were infected with S. typhimurium expressing mCherry for 1 h at an MOI of 10 and then washed. The samples were fixed, and the GFP-LC3 association rate was examined by fluorescence microscopy. The average ± SD is shown for three independent experiments where at least 100 bacteria were counted. Scale bar: 2 μm. (D−I) MEFs of the noted Atg KOs and their parental cells or NIH3T3 cells with or without Atg4BC74A expression were infected with S. typhimurium for 10 min in DMEM without antibiotics at an MOI of 100. After infection, the cells were washed to remove extracellular S. typhimurium and incubated in DMEM containing gentamicin for 6 h. The cell lysates were plated on LB plates and the number of colonies was counted after a 1-d incubation at 37°C. The average ± SD of three independent experiments is shown.
FIGURE 2:
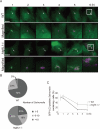
Extended trace of GFP-LC3 association with Salmonella. (A) Wild-type or Atg9L1 KO MEFs stably expressing GFP-LC3 were infected with S. typhimurium expressing mCherry for 5 min at an MOI of 100, washed, and then incubated for 1 h. One GFP-LC3–positive S. typhimurium was followed by time-lapse imaging at 4-min intervals with the automated trace system of MetaMorph until 6 h postinfection. The elapsed time is indicated. Scale bar: 20 μm. (B) The final number of S. typhimurium in each cell was determined after 6 h of time-lapse imaging and grouped as indicated. (C) Cells stably expressing GFP-LC3 were infected with S. typhimurium expressing mCherry and incubated. The cells were fixed at the indicated time points and examined by fluorescence microscopy. At least 100 cells were counted for each experiment. The average ± SD is shown for three independent experiments.
FIGURE 3:
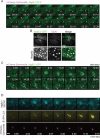
Live-cell imaging of Atg9L1 localization. (A and C) MEFs stably expressing Atg9L1-GFP were infected with S. typhimurium expressing mCherry for 5 min. The cells were washed and images were then taken using a fluorescence inverted microscope at ∼3-s intervals for 4 min or at ∼50-s intervals for 28 min. The elapsed time is indicated. Scale bar: 5 μm (A) or 1 μm (C). (B) MEFs stably expressing Atg9L1-GFP were fixed and immunostained with anti-EEA1 antibodies. Scale bar: 20 μm. (D) MEFs stably expressing Atg9L1-SECFP and EYFP-LC3 were infected with S. typhimurium expressing mCherry for 5 min at an MOI of 100 and images were taken as in (A) and (C) at ∼30-s intervals for 10 min. Scale bar: 1 μm.
FIGURE 4:
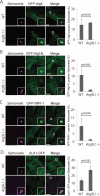
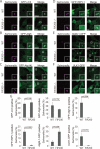
Localization of Atg proteins in Atg9L1 KO cells. Atg9L1 KO MEFs and the parental wild-type cells stably expressing GFP-Atg5 (A), GFP-Atg14L (B), GFP-WIPI-1 (C), or ULK1-GFP (D) were infected with S. typhimurium expressing mCherry for 1 h. After fixation, images were taken. The number of Atg-positive bacteria for each condition was determined and the percentages per all bacteria are shown. The average ± SD is shown for three independent experiments where at least 100 bacteria were counted. Scale bar: 20 μm.
FIGURE 5:
The effects of wortmannin on the association of each Atg protein with S. typhimurium. MEFs stably expressing GFP-LC3 (A), GFP-Atg5 (B), or GFP-WIPI-1 (C) were treated with 100 nM wortmannin for 15 min prior to infection. The cells were infected with S. typhimurium for 30 min at an MOI of 40, washed, fixed, and then immunostained with anti-EEA1 antibodies and stained with Hoechst 33342. Scale bar: 2 μm. (D, E) MEFs stably expressing GFP-LC3 were infected with S. typhimurium expressing mCherry for 1 h at an MOI of 10 and then washed. The samples were fixed, and the GFP-LC3 association rate was examined by fluorescence microscopy. At least 100 bacteria were counted. Scale bar: 2 μm.
FIGURE 6:
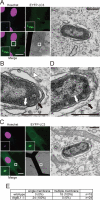
CLEM analysis of EYFP-LC3−associated membranes. Wild-type (A and B) or Atg9L1 KO MEFs (C and D) stably expressing EYFP-LC3 were cultured on gridded, glass bottom dishes and then infected with S. typhimurium for 30 min. The cells were fixed and stained with Hoechst 33342. GFP-LC3−positive S. typhimurium was observed using a confocal laser scanning microscope. The same specimens were further examined by EM. The electron micrographs were taken in the same sample field as the transmission electron microscope. White scale bar: 20 μm; black scale bar: 500 nm. (E) Quantification of the number of single or multiple membranes surrounding S. typhimurium in wild-type or Atg9L1 KO MEFs.
FIGURE 7:
Association of Atg proteins with S. typhimurium in FIP200 KO MEFs. Wild-type or FIP200 KO MEFs stably expressing GFP-LC3 (A), GFP-Atg5 (B), GFP-Atg14L (C), GFP-WIPI-1 (D), Atg9L1-GFP (E), or ULK1-GFP (F) were infected with S. typhimurium expressing mCherry for 1 h. The cells were fixed and observed. (G) The percentage of bacteria associated with the Atg proteins was determined by fluorescence microscopy. The average ± SD is shown for at least three independent experiments where at least 100 bacteria were examined. Scale bar: 20 μm.
FIGURE 8:
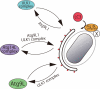
Model of Atg protein dynamics in Salmonella xenophagy. Atg14L, ULK1, and Atg9L1 cycle between the membrane formation site near S. typhimurium and another cellular pool. The cycling to and from the membrane formation site requires the indicated Atg proteins. S. typhimurium provides an unknown recruitment factor (X), and the Atg16L1 complex is potentially recruited near the SCV. The isolation membrane, formed independently of LC3, may be the preferable target of LC3 lipidation. In Atg9L1 KO MEFs, the isolation membrane does not form, and the SCV is the target of LC3 lipidation. Even without the LC3 system, an incomplete isolation membrane is formed.
Similar articles
- Three-Axis Model for Atg Recruitment in Autophagy against Salmonella.
Noda T, Kageyama S, Fujita N, Yoshimori T. Noda T, et al. Int J Cell Biol. 2012;2012:389562. doi: 10.1155/2012/389562. Epub 2012 Feb 28. Int J Cell Biol. 2012. PMID: 22505927 Free PMC article. - Activation of focal adhesion kinase by Salmonella suppresses autophagy via an Akt/mTOR signaling pathway and promotes bacterial survival in macrophages.
Owen KA, Meyer CB, Bouton AH, Casanova JE. Owen KA, et al. PLoS Pathog. 2014 Jun 5;10(6):e1004159. doi: 10.1371/journal.ppat.1004159. eCollection 2014 Jun. PLoS Pathog. 2014. PMID: 24901456 Free PMC article. - Role of ULK-FIP200 complex in mammalian autophagy: FIP200, a counterpart of yeast Atg17?
Hara T, Mizushima N. Hara T, et al. Autophagy. 2009 Jan;5(1):85-7. doi: 10.4161/auto.5.1.7180. Epub 2009 Jan 13. Autophagy. 2009. PMID: 18981720 - Molecular basis of canonical and bactericidal autophagy.
Noda T, Yoshimori T. Noda T, et al. Int Immunol. 2009 Nov;21(11):1199-204. doi: 10.1093/intimm/dxp088. Epub 2009 Sep 7. Int Immunol. 2009. PMID: 19737785 Review. - Autophagy basics.
Tanida I. Tanida I. Microbiol Immunol. 2011 Jan;55(1):1-11. doi: 10.1111/j.1348-0421.2010.00271.x. Microbiol Immunol. 2011. PMID: 21175768 Review.
Cited by
- Three-Axis Model for Atg Recruitment in Autophagy against Salmonella.
Noda T, Kageyama S, Fujita N, Yoshimori T. Noda T, et al. Int J Cell Biol. 2012;2012:389562. doi: 10.1155/2012/389562. Epub 2012 Feb 28. Int J Cell Biol. 2012. PMID: 22505927 Free PMC article. - Autophagy regulation through Atg9 traffic.
Reggiori F, Tooze SA. Reggiori F, et al. J Cell Biol. 2012 Jul 23;198(2):151-3. doi: 10.1083/jcb.201206119. J Cell Biol. 2012. PMID: 22826119 Free PMC article. - Bombyx mori Nuclear Polyhedrosis Virus (BmNPV) Induces Host Cell Autophagy to Benefit Infection.
Wang L, Xiao Q, Zhou XL, Zhu Y, Dong ZQ, Chen P, Pan MH, Lu C. Wang L, et al. Viruses. 2017 Dec 30;10(1):14. doi: 10.3390/v10010014. Viruses. 2017. PMID: 29301200 Free PMC article. - Self and nonself: how autophagy targets mitochondria and bacteria.
Randow F, Youle RJ. Randow F, et al. Cell Host Microbe. 2014 Apr 9;15(4):403-11. doi: 10.1016/j.chom.2014.03.012. Cell Host Microbe. 2014. PMID: 24721569 Free PMC article. Review.
References
- Bakowski MA, Braun V, Brumell JH. Salmonella-containing vacuoles: directing traffic and nesting to grow. Traffic. 2008;9:2022–2031. - PubMed
- Birmingham CL, Smith AC, Bakowski MA, Yoshimori T, Brumell JH. Autophagy controls Salmonella infection in response to damage to the Salmonella-containing vacuole. J Biol Chem. 2006;281:11374–11383. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases
Miscellaneous