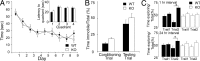Impairment of select forms of spatial memory and neurotrophin-dependent synaptic plasticity by deletion of glial aquaporin-4 - PubMed (original) (raw)
Impairment of select forms of spatial memory and neurotrophin-dependent synaptic plasticity by deletion of glial aquaporin-4
Vanessa A Skucas et al. J Neurosci. 2011.
Abstract
Aquaporin-4 (AQP4) is the major water channel in the CNS and is primarily expressed in astrocytes. Little is known about the potential for AQP4 to influence synaptic plasticity, although many studies have shown that it regulates the response of the CNS to injury. Therefore, we evaluated long-term potentiation (LTP) and long-term depression (LTD) in AQP4 knock-out (KO) and wild-type mice. KO mice exhibited a selective defect in LTP and LTD without a change in basal transmission or short-term plasticity. Interestingly, the impairment in LTP in KO mice was specific for the type of LTP that depends on the neurotrophin BDNF, which is induced by stimulation at theta rhythm [theta-burst stimulation (TBS)-LTP], but there was no impairment in a form of LTP that is BDNF independent, induced by high-frequency stimulation. LTD was also impaired in KO mice, which was rescued by a scavenger of BDNF or blockade of Trk receptors. TrkB receptors, which mediate effects of BDNF on TBS-LTP, were not altered in KO mice, but p75NTR, the receptor that binds all neurotrophins and has been implicated in some types of LTD, was decreased. The KO mice also exhibited a cognitive defect, which suggests a new role for AQP4 and astrocytes in normal cognitive function. This defect was evident using a test for location-specific object memory but not Morris water maze or contextual fear conditioning. The results suggest that AQP4 channels in astrocytes play an unanticipated role in neurotrophin-dependent plasticity and influence behavior.
Figures
Figure 1.
Selective impairments in synaptic plasticity in AQP4 KO mice. A1, Superimposed fEPSPs evoked with increasing stimulus intensities (by increasing the duration of the stimulus, in microseconds) show similar responses in WT and KO mice. A2, Quantified fEPSP slopes show similarity of WT and KO mice. A3, Similarity of paired-pulse facilitation of fEPSP slope (half-maximal stimuli) in WT and KO mice. B, Top, Similar sPSCs in WT and KO pyramidal cells. Bottom, Cumulative frequency plots did not demonstrate differences in genotypes. C, Comparison of TBS-LTP in WT (black) and KO (white) mice. D, Comparison of LTD in WT and KO mice. E, Comparison of HFS-LTP in WT and KO mice. Arrows denote TBS and HFS; LFS is marked by the bar. Additional data are in the text; also, see Notes.
Figure 2.
Selective OP deficit in KO mice. A, In the MWM, there were no significant differences in the average time to locate the hidden platform, plotted for each day animals were tested. Inset, There were no differences between genotypes in the probe trial (1, 2, 3, and 4 reflect each quadrant; 4 = target quadrant; see Notes). Note that CD1 mice normally do not exhibit a preference for the target quadrant in the probe trial (Adams et al., 2002). B, In CFC, there were no significant differences between WT and KO mice in the conditioning or testing trials. C, KO mice were impaired in OP because they did not spend more time in trial 2 exploring the moved object, but WT mice did.
Figure 3.
Neurotrophin signaling in WT and KO mice. A1,TrkB-Fc rescued LTD in KO mice. Slices from KO mice were exposed (thick bar) to TrkB-Fc or control IgG for 15 min before and after LFS. A2, K252a rescued LTD in KO mice. Experiments analogous to those in A were conducted, except the slices were exposed to K252a (300 n
m
) or vehicle (0.05% DMSO). B1, Full-length (F) and truncated (T) TrkB levels were not significantly different in WT and KO mice. B2, WT mice had reduced p75NTR receptor levels.
Similar articles
- Aquaporin-4 water channels and synaptic plasticity in the hippocampus.
Scharfman HE, Binder DK. Scharfman HE, et al. Neurochem Int. 2013 Dec;63(7):702-11. doi: 10.1016/j.neuint.2013.05.003. Epub 2013 May 15. Neurochem Int. 2013. PMID: 23684954 Free PMC article. Review. - Chronic ceftriaxone treatment rescues hippocampal memory deficit in AQP4 knockout mice via activation of GLT-1.
Yang J, Li MX, Luo Y, Chen T, Liu J, Fang P, Jiang B, Hu ZL, Jin Y, Chen JG, Wang F. Yang J, et al. Neuropharmacology. 2013 Dec;75:213-22. doi: 10.1016/j.neuropharm.2013.08.009. Epub 2013 Aug 22. Neuropharmacology. 2013. PMID: 23973312 - The neurotrophin-inducible gene Vgf regulates hippocampal function and behavior through a brain-derived neurotrophic factor-dependent mechanism.
Bozdagi O, Rich E, Tronel S, Sadahiro M, Patterson K, Shapiro ML, Alberini CM, Huntley GW, Salton SR. Bozdagi O, et al. J Neurosci. 2008 Sep 24;28(39):9857-69. doi: 10.1523/JNEUROSCI.3145-08.2008. J Neurosci. 2008. PMID: 18815270 Free PMC article. - TrkB/BDNF-dependent striatal plasticity and behavior in a genetic model of epilepsy: modulation by valproic acid.
Ghiglieri V, Sgobio C, Patassini S, Bagetta V, Fejtova A, Giampà C, Marinucci S, Heyden A, Gundelfinger ED, Fusco FR, Calabresi P, Picconi B. Ghiglieri V, et al. Neuropsychopharmacology. 2010 Jun;35(7):1531-40. doi: 10.1038/npp.2010.23. Epub 2010 Mar 3. Neuropsychopharmacology. 2010. PMID: 20200504 Free PMC article. - BDNF-induced local protein synthesis and synaptic plasticity.
Leal G, Comprido D, Duarte CB. Leal G, et al. Neuropharmacology. 2014 Jan;76 Pt C:639-56. doi: 10.1016/j.neuropharm.2013.04.005. Epub 2013 Apr 16. Neuropharmacology. 2014. PMID: 23602987 Review.
Cited by
- Cognitive Impairment in Neuromyelitis Optica Spectrum Disorders: A Review of Clinical and Neuroradiological Features.
Oertel FC, Schließeit J, Brandt AU, Paul F. Oertel FC, et al. Front Neurol. 2019 Jun 12;10:608. doi: 10.3389/fneur.2019.00608. eCollection 2019. Front Neurol. 2019. PMID: 31258505 Free PMC article. Review. - Glia protein aquaporin-4 regulates aversive motivation of spatial memory in Morris water maze.
Zhang J, Li Y, Chen ZG, Dang H, Ding JH, Fan Y, Hu G. Zhang J, et al. CNS Neurosci Ther. 2013 Dec;19(12):937-44. doi: 10.1111/cns.12191. Epub 2013 Oct 25. CNS Neurosci Ther. 2013. PMID: 24165567 Free PMC article. - Aquaporins in Brain Edema and Neuropathological Conditions.
Filippidis AS, Carozza RB, Rekate HL. Filippidis AS, et al. Int J Mol Sci. 2016 Dec 28;18(1):55. doi: 10.3390/ijms18010055. Int J Mol Sci. 2016. PMID: 28036023 Free PMC article. Review. - Fasudil attenuates lipopolysaccharide-induced cognitive impairment in C57BL/6 mice through anti-oxidative and anti-inflammatory effects: Possible role of aquaporin-4.
Jalalkamali S, Ghahremani M, Jashn V, Lajevardi NS, Koloor SM, Jazaeri SZ, Fahanik-Babaei J. Jalalkamali S, et al. IBRO Neurosci Rep. 2024 Oct 24;17:372-381. doi: 10.1016/j.ibneur.2024.10.004. eCollection 2024 Dec. IBRO Neurosci Rep. 2024. PMID: 39534317 Free PMC article. - Expression of the Astrocyte Water Channel Aquaporin-4 in the Mouse Brain.
Hubbard JA, Hsu MS, Seldin MM, Binder DK. Hubbard JA, et al. ASN Neuro. 2015 Oct 21;7(5):1759091415605486. doi: 10.1177/1759091415605486. Print 2015 Sep-Oct. ASN Neuro. 2015. PMID: 26489685 Free PMC article.
References
- Adams B, Fitch T, Chaney S, Gerlai R. Altered performance characteristics in cognitive tasks: comparison of the albino ICR and CD1 mouse strains. Behav Brain Res. 2002;133:351–361. - PubMed
- Akaneya Y, Tsumoto T, Hatanaka H. Brain-derived neurotrophic factor blocks long-term depression in rat visual cortex. J Neurophysiol. 1996;76:4198–4201. - PubMed
- Angelo MF, Aviles-Reyes RX, Villarreal A, Barker P, Reines AG, Ramos AJ. P75 NTR expression is induced in isolated neurons of the penumbra after ischemia by cortical devascularization. J Neurosci Res. 2009;87:1892–1903. - PubMed
- Assini FL, Duzzioni M, Takahashi RN. Object location memory in mice: Pharmacological validation and further evidence of hippocampal CA1 participation. Behav Brain Res. 2009;204:206–211. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- R01 MH081004/MH/NIMH NIH HHS/United States
- R01 NS030687/NS/NINDS NIH HHS/United States
- R01 NS064114/NS/NINDS NIH HHS/United States
- R37 DK035124/DK/NIDDK NIH HHS/United States
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases
Research Materials