sGC{alpha}1 mediates the negative inotropic effects of NO in cardiac myocytes independent of changes in calcium handling - PubMed (original) (raw)
sGC{alpha}1 mediates the negative inotropic effects of NO in cardiac myocytes independent of changes in calcium handling
Sharon M Cawley et al. Am J Physiol Heart Circ Physiol. 2011 Jul.
Abstract
In the heart, nitric oxide (NO) modulates contractile function; however, the mechanisms responsible for this effect are incompletely understood. NO can elicit effects via a variety of mechanisms including S-nitrosylation and stimulation of cGMP synthesis by soluble guanylate cyclase (sGC). sGC is a heterodimer comprised of a β(1)- and an α(1)- or α(2)-subunit. sGCα(1)β(1) is the predominant isoform in the heart. To characterize the role of sGC in the regulation of cardiac contractile function by NO, we compared left ventricular cardiac myocytes (CM) isolated from adult mice deficient in the sGC α(1)-subunit (sGCα(1)(-/-)) and from wild-type (WT) mice. Sarcomere shortening under basal conditions was less in sGCα(1)(-/-) CM than in WT CM. To activate endogenous NO synthesis from NO synthase 3, CM were incubated with the β(3)-adrenergic receptor (β(3)-AR) agonist BRL 37344. BRL 37344 decreased cardiac contractility in WT CM but not in sGCα(1)(-/-) myocytes. Administration of spermine NONOate, an NO donor compound, did not affect sarcomeric shortening in CM of either genotype; however, in the presence of isoproterenol, addition of spermine NONOate reduced sarcomere shortening in WT but not in sGCα(1)(-/-) CM. Neither BRL 37344 nor spermine NONOate altered calcium handling in CM of either genotype. These findings suggest that sGCα(1) exerts a positive inotropic effect under basal conditions, as well as mediates the negative inotropic effect of β(3)-AR signaling. Additionally, our work demonstrates that sGCα(1)β(1) is required for NO to depress β(1)/β(2)-AR-stimulated cardiac contractility and that this modulation is independent of changes in calcium handling.
Figures
Fig. 1.
Localization of soluble guanylate cyclase-α1 (sGCα1) in cardiac myocytes (CM). CM isolated from wild-type (WT; A_–_C and G_–_I) and sGCα1−/− (D_–_F and J_–_L) mice were reacted with primary antibodies recognizing sGCα1 (B and E), sGCβ1 (H and K), and α-actinin (A, D, G, and J), and bound antibody was visualized by confocal microscopy using fluorescently labeled secondary antibodies. α-Actinin was detected with FITC-labeled anti-mouse IgG (green), and sGCα1 and sGCβ1 were detected using Dylight 594-labeled anti-rabbit IgG (red). Merged view (C, F, I, and L) demonstrates that sGCα1 colocalizes with α-actinin on Z-lines. Scale bars (white) = 10 μm.
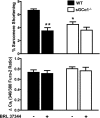
Fig. 2.
Effect of BRL 37344 on WT and sGCα1−/− CM contractility and calcium handling. %Sarcomere shortening (top) and calcium transient amplitude (ΔCai; bottom) are shown for WT and sGCα1−/− CM incubated in the absence and presence of BRL 37344 (1 nM). CM were incubated for 10 min and perfused with Tyrode buffer containing 1 nM of the β3-AR agonist BRL 37344. Cells were paced at 2 Hz. *P < 0.001 for WT BRL vs. WT basal. **P < 0.01 for sGCα1−/− basal vs. WT basal.
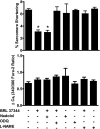
Fig. 3.
Effect of nadolol, nitro-
l
-arginine methyl ester (
l
-NAME), and 1H-(1,2,4)oxadiazolo(4,3-a)-quinoxalin-1-one (ODQ) on contractile function and calcium handling after β3-adrenergic receptor stimulation in WT CM. %Sarcomere shortening (top) and calcium transient amplitude (bottom) from WT CM incubated with BRL 37344 and 10 μM nadolol, 10 mM
l
-NAME, or 10 μM ODQ and paced at 2 Hz. *P value of < 0.01 for indicated groups vs. WT basal in Bonferroni posttests.
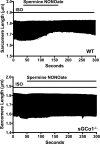
Fig. 4.
Effect of nitric oxide on β-adrenergic receptor-stimulated sarcomere shortening in WT and sGCα1−/− CM. Representative tracing demonstrating time-dependent change in sarcomere length of a single isoproterenol (ISO)-stimulated, paced WT (top) and sGCα1−/− (bottom) CM during perfusion with 100 μM spermine NONOate.
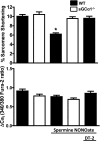
Fig. 5.
Summary data of %sarcomere shortening and corresponding Ca2+ transient peak height amplitudes of WT and sGCα1−/− CM. All CM were preincubated with ISO (10 nM) for 10 min before imaging. Graphs represent percentage of sarcomere shortening (top) and Ca2+ transient amplitude (bottom) measured at 150 s after start of perfusion buffer with or without spermine NONOate (100 μM). Additional CM were preincubated and continuously perfused with 125 nM DT-2 where indicated before administration of spermine NONOate. *P < 0.05 WT vs. WT with spermine NONOate.
Similar articles
- Phosphodiesterase 5 restricts NOS3/Soluble guanylate cyclase signaling to L-type Ca2+ current in cardiac myocytes.
Wang H, Kohr MJ, Traynham CJ, Ziolo MT. Wang H, et al. J Mol Cell Cardiol. 2009 Aug;47(2):304-14. doi: 10.1016/j.yjmcc.2009.03.021. Epub 2009 Apr 1. J Mol Cell Cardiol. 2009. PMID: 19345227 Free PMC article. - In vitro and in vivo studies on the importance of the soluble guanylyl cyclase α1 subunit in penile erection.
Decaluwé K, Nimmegeers S, Thoonen R, Buys E, Brouckaert P, Van de Voorde J. Decaluwé K, et al. World J Urol. 2010 Oct;28(5):643-50. doi: 10.1007/s00345-010-0509-7. Epub 2010 Jan 23. World J Urol. 2010. PMID: 20098992 - Functional role of the soluble guanylyl cyclase alpha(1) subunit in vascular smooth muscle relaxation.
Nimmegeers S, Sips P, Buys E, Brouckaert P, Van de Voorde J. Nimmegeers S, et al. Cardiovasc Res. 2007 Oct 1;76(1):149-59. doi: 10.1016/j.cardiores.2007.06.002. Epub 2007 Jun 12. Cardiovasc Res. 2007. PMID: 17610859 - Recent advances in cardiac beta(2)-adrenergic signal transduction.
Xiao RP, Cheng H, Zhou YY, Kuschel M, Lakatta EG. Xiao RP, et al. Circ Res. 1999 Nov 26;85(11):1092-100. doi: 10.1161/01.res.85.11.1092. Circ Res. 1999. PMID: 10571541 Review. - Beta-3 adrenoceptors as new therapeutic targets for cardiovascular pathologies.
Gauthier C, Rozec B, Manoury B, Balligand JL. Gauthier C, et al. Curr Heart Fail Rep. 2011 Sep;8(3):184-92. doi: 10.1007/s11897-011-0064-6. Curr Heart Fail Rep. 2011. PMID: 21633786 Review.
Cited by
- Fractalkine depresses cardiomyocyte contractility.
Taube D, Xu J, Yang XP, Undrovinas A, Peterson E, Harding P. Taube D, et al. PLoS One. 2013 Jul 30;8(7):e69832. doi: 10.1371/journal.pone.0069832. Print 2013. PLoS One. 2013. PMID: 23936109 Free PMC article. - Acute hemodynamic effects of riociguat in patients with pulmonary hypertension associated with diastolic heart failure (DILATE-1): a randomized, double-blind, placebo-controlled, single-dose study.
Bonderman D, Pretsch I, Steringer-Mascherbauer R, Jansa P, Rosenkranz S, Tufaro C, Bojic A, Lam CSP, Frey R, Ochan Kilama M, Unger S, Roessig L, Lang IM. Bonderman D, et al. Chest. 2014 Nov;146(5):1274-1285. doi: 10.1378/chest.14-0106. Chest. 2014. PMID: 24991733 Free PMC article. Clinical Trial. - Dynamic denitrosylation via S-nitrosoglutathione reductase regulates cardiovascular function.
Beigi F, Gonzalez DR, Minhas KM, Sun QA, Foster MW, Khan SA, Treuer AV, Dulce RA, Harrison RW, Saraiva RM, Premer C, Schulman IH, Stamler JS, Hare JM. Beigi F, et al. Proc Natl Acad Sci U S A. 2012 Mar 13;109(11):4314-9. doi: 10.1073/pnas.1113319109. Epub 2012 Feb 24. Proc Natl Acad Sci U S A. 2012. PMID: 22366318 Free PMC article.
References
- Barouch LA, Harrison RW, Skaf MW, Rosas GO, Cappola TP, Kobeissi ZA, Hobai IA, Lemmon CA, Burnett AL, O'Rourke B, Rodriguez ER, Huang PL, Lima JA, Berkowitz DE, Hare JM. Nitric oxide regulates the heart by spatial confinement of nitric oxide synthase isoforms. Nature 416: 337–339, 2002 - PubMed
- Boivin B, Lavoie C, Vaniotis G, Baragli A, Villeneuve LR, Ethier N, Trieu P, Allen BG, Hebert TE. Functional beta-adrenergic receptor signaling on nuclear membranes in adult rat and mouse ventricular cardiomyocytes. Cardiovasc Res 71: 69–78, 2006 - PubMed
- Borlaug BA, Melenovsky V, Marhin T, Fitzgerald P, Kass DA. Sildenafil inhibits beta-adrenergic-stimulated cardiac contractility in humans. Circulation 112: 2642–2649, 2005 - PubMed
- Budworth J, Meillerais S, Charles I, Powell K. Tissue distribution of the human soluble guanylate cyclases. Biochem Biophys Res Commun 263: 696–701, 1999 - PubMed
- Buys ES, Sips P, Vermeersch P, Raher MJ, Rogge E, Ichinose F, Dewerchin M, Bloch KD, Janssens S, Brouckaert P. Gender-specific hypertension and responsiveness to nitric oxide in sGCalpha1 knockout mice. Cardiovasc Res 79: 179–186, 2008 - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Research Materials