Analysis of the anti-tumor effect of cetuximab using protein kinetics and mouse xenograft models - PubMed (original) (raw)
Analysis of the anti-tumor effect of cetuximab using protein kinetics and mouse xenograft models
Teppei Matsuo et al. BMC Res Notes. 2011.
Abstract
Background: The binding of EGFR and its ligands leads to autophosphorylation of receptor tyrosine kinase as well as subsequent activation of signal transduction pathways that are involved in regulating cellular proliferation, differentiation, and survival. An EGFR inhibitor, cetuximab binds to EGFR and consequently blocks a variety of cellular processes. KRAS/BRAF mutations are known to be associated with a low response rate to cetuximab. In the present study, to clarify the anti-tumor mechanisms of cetuximab, we evaluated the KRAS/BRAF status, phosphorylation level of the EGFR pathway, and the tumor suppression effect in vivo, using a human colon cancer cell line HT29, which exhibited the highest EGFR expression in response to the cetuximab therapy among the 6 colorectal cancer cell lines tested.
Findings: The conventional growth suppression assay did not work efficiently with cetuximab. EGF, TGF-α, and IGF activated the EGFR/MAPK cell signaling pathway by initiating the phosphorylation of EGFR. Cetuximab partially inhibited the EGFR/MAPK pathway induced by EGF, TGF-α, and IGF. However, cetuximab exposure induced the EGFR, MEK, and ERK1/2 phosphorylation by itself. Mouse xenograft tumor growth was significantly inhibited by cetuximab and both cetuximab-treated and -untreated xenograft specimens exhibited phosphorylations of the EGFR pathway proteins.
Conclusions: We have confirmed that cetuximab inhibited the EGFR/MAPK pathway and reduced tumor growth in the xenografts while the remaining tumor showed EGFR pathway activation. These results suggest that: ( i ) The effect of cetuximab in growth signaling is not sufficient to induce complete growth suppression in vitro; ( ii ) time-course monitoring may be necessary to evaluate the effect of cetuximab because EGFR signaling is transmitted in a minute order; and ( iii ) cetuximab treatment may have cells acquired resistant selectively survived in the heterogeneous cancer population.
Figures
Figure 1
Characterization of the colorectal cancer cell lines. Protein expression of EGFR in colorectal cancer cell lines and sequence histograms of HT29, A, Expression of EGFR in HT29, CW2, HCT116, JHSK-rec, JHCOLO-YI and TT1TKB cells determined by Western blot. Pan-Actin was used as a loading control. B, Sequencing analysis of KRAS and the BRAF gene in HT29 cells.
Figure 2
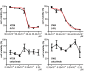
Growth suppression assay for in vitro antitumor activity of cetuximab in CRC cell lines. Growth suppression curves resulting from either 5-FU or cetuximab dose-dependent administrations for 48h are shown. The error bar shows mean ± SEM (standard error of the mean) of each concentration data point. The vertical axis represents the cell viability, and the horizontal axis represents the drug concentration.
Figure 3
Kinetics of proteins involved in EGFR/MAPK signaling with cetuximab in HT29 cells. A, HT29 cells were treated with serum-free RPMI for 24 hours, followed by the addition of EGF 100ng/ml, TGF-α 50ng/ml, IGF 50ng/ml, and cetuximab 300ng/ml, resulting in a total of 7 conditions for 0, 1, 5, 10, 15 minutes before collections of protein lysate. The phosphorylation of EGFR, MEK, and ERK was visualized using Western blot analysis. Actin was used as a loading control. B, A Western blot band was plotted in an arbitrary unit showing the time course.
Figure 4
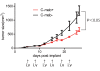
In vivo tumor suppression by cetuximab. Growth suppression of the HT29 mouse xenograft is present. HT29 xenografts were either untreated or treated with 200 μg of cetuximab per mouse twice a week. The tumor volume was measured every 2-3 days, and all mice were sacrificed after 26 days of treatment. Error bars represent the standard error of the mean. We compared the tumor volume of treated and untreated xenografts using the student t-test (p < 0.05).
Figure 5
Immunohistochemistry of xenograft tumors. HT29 mouse xenograft tumors were removed and stained after cetuximab treatment. Tumor sections were stained with HE, anti-EGFR, anti-pEGFR(1045), anti-pERK(Thr202/Tyr204). Images are from cetuximab-treated (top) and -untreated (bottom).
Figure 6
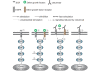
Possible EGFR signaling pathways affected by cetuximab. ➀EGF activates EGFR/MAPK signaling. ➁ Phosphorylation of downstream signaling is induced more quickly by TGF-α and IGF than EGF. ➂Potential negative-feedback from downstream signals. ➃Cetuximab reduces the phosphorylation level induced by the three growth factors. ➄Cetuximab binding to the receptor induces the receptor dimerization resulting in protein phosphorylation of downstream proteins. However, inhibition of the receptor internalization leads to the suppression of protein phosphorylation in downstream proteins (as a consequence, in the presence of cetuximab, the level of protein phosphorylation does exist but at a very low level).
Similar articles
- Primary and acquired resistance of colorectal cancer cells to anti-EGFR antibodies converge on MEK/ERK pathway activation and can be overcome by combined MEK/EGFR inhibition.
Troiani T, Napolitano S, Vitagliano D, Morgillo F, Capasso A, Sforza V, Nappi A, Ciardiello D, Ciardiello F, Martinelli E. Troiani T, et al. Clin Cancer Res. 2014 Jul 15;20(14):3775-86. doi: 10.1158/1078-0432.CCR-13-2181. Epub 2014 May 8. Clin Cancer Res. 2014. PMID: 24812410 - Increased TGF-α as a mechanism of acquired resistance to the anti-EGFR inhibitor cetuximab through EGFR-MET interaction and activation of MET signaling in colon cancer cells.
Troiani T, Martinelli E, Napolitano S, Vitagliano D, Ciuffreda LP, Costantino S, Morgillo F, Capasso A, Sforza V, Nappi A, De Palma R, D'Aiuto E, Berrino L, Bianco R, Ciardiello F. Troiani T, et al. Clin Cancer Res. 2013 Dec 15;19(24):6751-65. doi: 10.1158/1078-0432.CCR-13-0423. Epub 2013 Oct 11. Clin Cancer Res. 2013. PMID: 24122793 - Receptor tyrosine kinase-dependent PI3K activation is an escape mechanism to vertical suppression of the EGFR/RAS/MAPK pathway in KRAS-mutated human colorectal cancer cell lines.
Vitiello PP, Cardone C, Martini G, Ciardiello D, Belli V, Matrone N, Barra G, Napolitano S, Della Corte C, Turano M, Furia M, Troiani T, Morgillo F, De Vita F, Ciardiello F, Martinelli E. Vitiello PP, et al. J Exp Clin Cancer Res. 2019 Jan 28;38(1):41. doi: 10.1186/s13046-019-1035-0. J Exp Clin Cancer Res. 2019. PMID: 30691487 Free PMC article. - Cetuximab: an epidermal growth factor receptor chemeric human-murine monoclonal antibody.
Harding J, Burtness B. Harding J, et al. Drugs Today (Barc). 2005 Feb;41(2):107-27. doi: 10.1358/dot.2005.41.2.882662. Drugs Today (Barc). 2005. PMID: 15821783 Review. - Integration of novel agents in the treatment of colorectal cancer.
Iqbal S, Lenz HJ. Iqbal S, et al. Cancer Chemother Pharmacol. 2004 Sep;54 Suppl 1:S32-9. doi: 10.1007/s00280-004-0884-0. Cancer Chemother Pharmacol. 2004. PMID: 15309512 Review.
Cited by
- Function characterization of a glyco-engineered anti-EGFR monoclonal antibody cetuximab in vitro.
Yi CH, Ruan CP, Wang H, Xu XY, Zhao YP, Fang M, Ji J, Gu X, Gao CF. Yi CH, et al. Acta Pharmacol Sin. 2014 Nov;35(11):1439-46. doi: 10.1038/aps.2014.77. Epub 2014 Sep 29. Acta Pharmacol Sin. 2014. PMID: 25263334 Free PMC article. - Expression of Epidermal Growth Factor Receptor Detected by Cetuximab Indicates Its Efficacy to Inhibit In Vitro and In Vivo Proliferation of Colorectal Cancer Cells.
Shigeta K, Hayashida T, Hoshino Y, Okabayashi K, Endo T, Ishii Y, Hasegawa H, Kitagawa Y. Shigeta K, et al. PLoS One. 2013 Jun 18;8(6):e66302. doi: 10.1371/journal.pone.0066302. Print 2013. PLoS One. 2013. PMID: 23824671 Free PMC article. - Metronomic oral topotecan prolongs survival and reduces liver metastasis in improved preclinical orthotopic and adjuvant therapy colon cancer models.
Hackl C, Man S, Francia G, Milsom C, Xu P, Kerbel RS. Hackl C, et al. Gut. 2013 Feb;62(2):259-71. doi: 10.1136/gutjnl-2011-301585. Epub 2012 Apr 28. Gut. 2013. PMID: 22543158 Free PMC article. - Disseminated Mycobacterium chelonae infection in a patient receiving an epidermal growth factor receptor inhibitor for advanced head and neck cancer.
Bark CM, Traboulsi RS, Honda K, Starnes AM, Jacobs MR, Rodriguez B. Bark CM, et al. J Clin Microbiol. 2012 Jan;50(1):194-5. doi: 10.1128/JCM.05399-11. Epub 2011 Oct 26. J Clin Microbiol. 2012. PMID: 22031707 Free PMC article. - Theranostic potential of self-luminescent branched polyethyleneimine-coated superparamagnetic iron oxide nanoparticles.
Khodadust R, Unal O, Yagci Acar H. Khodadust R, et al. Beilstein J Nanotechnol. 2022 Jan 18;13:82-95. doi: 10.3762/bjnano.13.6. eCollection 2022. Beilstein J Nanotechnol. 2022. PMID: 35116215 Free PMC article.
References
- Herbst RS. Review of epidermal growth factor receptor biology. Int J Radiat Oncol Biol Phys. 2004;59(2 Suppl):21–6. - PubMed
- Goldstein NI. et al.Biological efficacy of a chimeric antibody to the epidermal growth factor receptor in a human tumor xenograft model. Clin Cancer Res. 1995;1(11):1311–8. - PubMed
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous