Tumor-associated macrophages recruit CCR6+ regulatory T cells and promote the development of colorectal cancer via enhancing CCL20 production in mice - PubMed (original) (raw)
Tumor-associated macrophages recruit CCR6+ regulatory T cells and promote the development of colorectal cancer via enhancing CCL20 production in mice
Jinlin Liu et al. PLoS One. 2011.
Abstract
Background: Tumor-associated macrophages (TAMs) remodel the colorectal cancer (CRC) microenvironment. Yet, findings on the role of TAMs in CRC seem to be contradictory compared with other cancers. FoxP3(+) regulatory T (Treg)-cells dominantly infiltrate CRC. However, the underlying molecular mechanism in which TAMs may contribute to the trafficking of Treg-cells to the tumor mass remains unknown.
Methodology/principal findings: CRC was either induced by N-methyl-N-nitrosourea (MNU) and H. pylori or established by subcutaneous injection of mouse colorectal tumor cell line (CMT93) in mice. CMT93 cells were co-cultured with primary macrophages in a transwell apparatus. Recruitment of FoxP3 green fluorescence protein positive (FoxP3(GFP+)) Treg-cells was assessed using the IVIS Imaging System or immunofluorescence staining. A role for macrophages in trafficking of Treg-cells and in the development of CRC was investigated in CD11b diphtheria toxin receptor (CD11b-DTR) transgenic C57BL/6J mice in which macrophages can be selectively depleted. Treg-cells remarkably infiltrated solid tumor, and predominantly expressed the homing chemokine receptor (CCR) 6 in the induced CRC model. Both CMT93 cancer cells and macrophages produced a large amount of CCL20, the sole ligand of CCR6 in vitro and in vivo. Injection of recombinant mouse CCL20 into tumor sites promoted its development with a marked recruitment of Treg-cells in the graft CRC model. Conditional macrophage ablation decreased CCL20 levels, blocked Treg-cell recruitment and inhibited tumor growth in CD11b-DTR mice grafted with CMT93.
Conclusions/significance: TAMs recruit CCR6(+) Treg-cells to tumor mass and promote its development via enhancing the production of CCL20 in a CRC mouse model.
Conflict of interest statement
Competing Interests: The authors have declared that no competing interests exist.
Figures
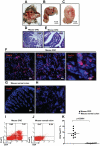
Figure 1. Increased numbers of FoxP3+ Treg-cells in tumor-infiltrating lymphocytes of CRC induced by MNU and H. pylori. in mice.
(A) Eighty weeks old C57BL/6J mouse with large CRC induced by MNU and H. pylori. (B) Higher magnification of the area indicated by the triangle (Δ) of (A). (C) Transect of the area indicated by the asterisk (*) of (B). (D) H&E staining of colorectal carcinoma tissue derived from CRC induced by MNU and H. pylori (original magnification, ×20). (E) Higher magnification of (D) as indicated by the rectangle. To examine FoxP3+ Treg-cells in mice with CRC induced by MNU and H. pylori, immunofluorescence staining of FoxP3 was performed on cryosections from mouse CRC or normal colon tissue. (F) Expression of FoxP3 (Red staining) in mouse CRC (original magnification, ×20, ×40 and ×100). (G) Negative expression of FoxP3 in mouse colon tissue (original magnification, ×20). (H) Rat IgG control staining (original magnification, ×20). Lymphocytes infiltrating CRC (n = 10) and normal mucosa (n = 6) were isolated for Flow cytometric analysis of FoxP3 expression. (I, J) The frequency of Treg-cells in CRC derived from eighty weeks old C57BL/6J mouse and normal mucosa in mice. (K) A significant increase in the frequency of Treg-cells was found in TIL of CRC compared with normal mucosa (p<0.0001 by Student's t Test). Representative data are shown which had been reproduced in 3 independent experiments.
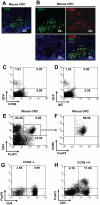
Figure 2. Requirement of CCR6 for trafficking of Treg-cells to CRC in mice.
Treg-cells (0.5–1×106) purified from spleens of FoxP3GFP mice were adoptively transferred into mice bearing CRC induced by MNU and H. pylori. (A) Cryosections from tumor were stained for cell nuclei with DAPI to investigate infiltration of FoxP3GFP+ Treg-cells in tumor mass (original magnification, ×20). (B) Co-localization of GFP with CCR6 staining. (C, D) Flow cytometric analysis for CCR6 of FoxPGFP+ Treg-cells derived from peripheral blood of naïve FoxP3GFP mice. (E, F) Flow cytometric analysis for CCR6 of infiltrating Treg-cells derived from CRC induced by MNU and H. pylori in mice. To confirm whether CCR6 is required for FoxP3+ Treg-cell trafficking, CMT93 cell line was grafted into CCR6−/− and CCR6+/+ mice. (G, H) Flow cytometric analysis of tumor-infiltrating CD4+FoxP3+ Treg-cells two weeks after CMT93 cell line grafting in CCR6−/− (G) and CCR6+/+ (H) mice. Representative data are shown which had been reproduced in 4 independent experiments.
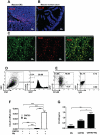
Figure 3. Contribution of TAMs to CCL20 production in CRC in mice.
(A, B) Cryosections from mouse CRC induced by MNU and H. pylori or mouse normal colon were stained for CCL20 (red, original magni-fication, ×20). Double immunostaining with anti-mouse CCL20 and F4/80 mAb was performed on cryosections derived from mouse CRC. (C) Co-localization of CCL20 with F4/80 staining (original magnification, ×20). (D) CMT93 was transducted with GFP, and confirmed by flow cytometry. (E) Flow cytometric analysis of CCL20+ cells three weeks after GFP+ CMT93 cells grafting in C57BL/6J mice. (F) mRNA levels of CCL20 for co-culture of mouse peritoneal macrophages with CMT93 cancer cells. AU, arbitrary units. (G) Protein levels of CCL20 for co-culture of mouse peritoneal macrophages with CMT93 cancer cells. Representative data are shown which had been reproduced in at least 2 independent experiments. * p<0.05, ** p<0.01,*** p<0.001, Student's t test.
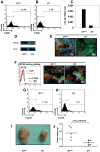
Figure 4. CCL20 recruited FoxP3+ Treg-cells to tumor mass and stimulated the growth of CRC in mice.
Mouse colorectal cell line, CMT 93 (1×106) with or without 0.5 µg mouse recombinant mouse CCL20 in 100 µl PBS were injected s.c. into FoxP3GFP mice, and 0.5 µg recombinant mouse CCL20 in 100 µl PBS was injected s.c. weekly for 28 days. Control FoxP3GFP mice were only given 100 µl PBS injection. (A) Normal FoxP3GFP mouse was used as a technique control. (B, C) Green fluorescence of FoxP3GFP+ cells in mice treated PBS or recombinant mouse CCL20 (white arrow) were monitored using the IVIS Imaging System at day 28. (D) Numbers of tumor-infiltrating FoxP3GFP+ Treg-cells derived from grafted CRC in mice treated with PBS or recombinant mouse CCL20 were analyzed by Flow cytometry. (E) The sizes of tumors were measured with calipers at the indicated time points for 28 days. Injection of recombinant mouse CCL20 resulted in a significant increase in tumor sizes (n = 5). (F) Expression levels of CCR6 by grafted CRC in CCR6−/− mice. (G) CMT 93 (1×106) with or without 0.5 µg mouse recombinant mouse CCL20 in 100 µl PBS were injected s.c. into CCR6−/− mice, and 0.5 µg recombinant mouse CCL20 in 100 µl PBS or 100 µl PBS was injected s.c. at every second day for 21 days. Tumor weight of CRC was measured at the end of experiment. Representative data are shown which had been reproduced in 3 independent experiments. * p<0.05, ** p<0.01, Student's t test.
Figure 5. Conditional macrophage ablation disrupted Treg-cell recruitment and inhibited the growth of CRC in mice.
(A, B) Flow cytometric analysis of TAMs derived from grafted CRC in CD11b-DTR mice injected with DTmu or DT. (C) CCL20 mRNA levels in grafted CRC in CD11b-DTR mice injected with DTmu or DT (data from 5 mice of each group were pooled together). (D) Lysates of grafted CRC from CD11b-DTR mice injected with DTmu or DT were subjected to western blotting for CCL20 expression. Actin - loading control. (E) Immunofluorescence staining with anti-mouse CCL20 was performed on cryosections of GFP+ CMT93 grafted tumor mass derived from CD11b-DTR mice injected with DTmu or DT for 14 days. (F) Flow cytometric analysis of CCR6 expression for GFP+ CMT93 cells before grafting (left panel), and CCR6 staining on cryosections of GFP+ CMT93 grafted tumor mass derived from CD11b-DTR mice injected with DTmu or DT for 14 days (middle and right panel). (G, H) Number of tumor-infiltrating FoxP3+ Treg-cells in grafted CRC in CD11b-DTR mice injected with DTmu or DT. (I) CRC grafted in CD11b-DTR mice injected with DTmu or DT for 14 days. (J) Tumor weight of CRC grafted in CD11b-DTR mice injected with DTmu or DT for 14 days. Representative data are shown which had been reproduced in 2 independent experiments.
Similar articles
- CCL20/CCR6-mediated migration of regulatory T cells to the Helicobacter pylori-infected human gastric mucosa.
Cook KW, Letley DP, Ingram RJ, Staples E, Skjoldmose H, Atherton JC, Robinson K. Cook KW, et al. Gut. 2014 Oct;63(10):1550-9. doi: 10.1136/gutjnl-2013-306253. Epub 2014 Jan 16. Gut. 2014. PMID: 24436142 Free PMC article. - Selective recruitment of regulatory T cell through CCR6-CCL20 in hepatocellular carcinoma fosters tumor progression and predicts poor prognosis.
Chen KJ, Lin SZ, Zhou L, Xie HY, Zhou WH, Taki-Eldin A, Zheng SS. Chen KJ, et al. PLoS One. 2011;6(9):e24671. doi: 10.1371/journal.pone.0024671. Epub 2011 Sep 14. PLoS One. 2011. PMID: 21935436 Free PMC article. - [Prophyromonas gingivalis Promotes the Formation of Immunosuppressive Microenvironment in Oral Squamous Cell Carcinoma by CCR6+ Regulatory T Cells: A Study of the Mechanisms Invovled].
Xu L, Tian X, Wang J, Zhang Y, Naijibai M, Ling B. Xu L, et al. Sichuan Da Xue Xue Bao Yi Xue Ban. 2025 Jan 20;56(1):191-197. doi: 10.12182/20250160105. Sichuan Da Xue Xue Bao Yi Xue Ban. 2025. PMID: 40109479 Free PMC article. Chinese. - Chemokine/chemokine receptor pair CCL20/CCR6 in human colorectal malignancy: An overview.
Frick VO, Rubie C, Keilholz U, Ghadjar P. Frick VO, et al. World J Gastroenterol. 2016 Jan 14;22(2):833-41. doi: 10.3748/wjg.v22.i2.833. World J Gastroenterol. 2016. PMID: 26811629 Free PMC article. Review. - The CCL20-CCR6 Axis in Cancer Progression.
Kadomoto S, Izumi K, Mizokami A. Kadomoto S, et al. Int J Mol Sci. 2020 Jul 22;21(15):5186. doi: 10.3390/ijms21155186. Int J Mol Sci. 2020. PMID: 32707869 Free PMC article. Review.
Cited by
- Natural and induced T regulatory cells in cancer.
Adeegbe DO, Nishikawa H. Adeegbe DO, et al. Front Immunol. 2013 Jul 11;4:190. doi: 10.3389/fimmu.2013.00190. eCollection 2013. Front Immunol. 2013. PMID: 23874336 Free PMC article. - Malignant B cells induce the conversion of CD4+CD25- T cells to regulatory T cells in B-cell non-Hodgkin lymphoma.
Han Y, Wu J, Bi L, Xiong S, Gao S, Yin L, Jiang L, Chen C, Yu K, Zhang S. Han Y, et al. PLoS One. 2011;6(12):e28649. doi: 10.1371/journal.pone.0028649. Epub 2011 Dec 9. PLoS One. 2011. PMID: 22174855 Free PMC article. - CASZ1 is a novel promoter of metastasis in ovarian cancer.
Wu YY, Chang CL, Chuang YJ, Wu JE, Tung CH, Chen YC, Chen YL, Hong TM, Hsu KF. Wu YY, et al. Am J Cancer Res. 2016 Jun 1;6(6):1253-70. eCollection 2016. Am J Cancer Res. 2016. PMID: 27429842 Free PMC article. - Cellular and Molecular Mechanisms of the Tumor Stroma in Colorectal Cancer: Insights into Disease Progression and Therapeutic Targets.
Shakhpazyan N, Mikhaleva L, Bedzhanyan A, Gioeva Z, Sadykhov N, Mikhalev A, Atiakshin D, Buchwalow I, Tiemann M, Orekhov A. Shakhpazyan N, et al. Biomedicines. 2023 Aug 23;11(9):2361. doi: 10.3390/biomedicines11092361. Biomedicines. 2023. PMID: 37760801 Free PMC article. Review. - New Approaches to Immunotherapy for HPV Associated Cancers.
Bergot AS, Kassianos A, Frazer IH, Mittal D. Bergot AS, et al. Cancers (Basel). 2011 Sep 2;3(3):3461-95. doi: 10.3390/cancers3033461. Cancers (Basel). 2011. PMID: 24212964 Free PMC article.
References
- Kamangar F, Dores GM, Anderson WF. Patterns of cancer incidence, mortality, and prevalence across five continents: defining priorities to reduce cancer disparities in different geographic regions of the world. J Clin Oncol. 2006;24:2137–2150. - PubMed
- Fontenot JD, Gavin MA, Rudensky AY. Foxp3 programs the development and function of CD4+CD25+ regulatory T cells. Nat Immunol. 2003;4:330–336. - PubMed
- Zou W. Regulatory T cells, tumour immunity and immunotherapy. Nat Rev Immunol. 2006;6:295–307. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials