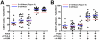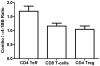Combination CTLA-4 blockade and 4-1BB activation enhances tumor rejection by increasing T-cell infiltration, proliferation, and cytokine production - PubMed (original) (raw)
Combination CTLA-4 blockade and 4-1BB activation enhances tumor rejection by increasing T-cell infiltration, proliferation, and cytokine production
Michael A Curran et al. PLoS One. 2011.
Abstract
Background: The co-inhibitory receptor Cytotoxic T-Lymphocyte Antigen 4 (CTLA-4) attenuates immune responses and prevent autoimmunity, however, tumors exploit this pathway to evade the host T-cell response. The T-cell co-stimulatory receptor 4-1BB is transiently upregulated on T-cells following activation and increases their proliferation and inflammatory cytokine production when engaged. Antibodies which block CTLA-4 or which activate 4-1BB can promote the rejection of some murine tumors, but fail to cure poorly immunogenic tumors like B16 melanoma as single agents.
Methodology/principal findings: We find that combining αCTLA-4 and α4-1BB antibodies in the context of a Flt3-ligand, but not a GM-CSF, based B16 melanoma vaccine promoted synergistic levels of tumor rejection. 4-1BB activation elicited strong infiltration of CD8+ T-cells into the tumor and drove the proliferation of these cells, while CTLA-4 blockade did the same for CD4+ effector T-cells. Anti-4-1BB also depressed regulatory T-cell infiltration of tumors. 4-1BB activation strongly stimulated inflammatory cytokine production in the vaccine and tumor draining lymph nodes and in the tumor itself. The addition of CTLA-4 blockade further increased IFN-γ production from CD4+ effector T-cells in the vaccine draining node and the tumor. Anti 4-1BB treatment, with or without CTLA-4 blockade, induced approximately 75% of CD8+ and 45% of CD4+ effector T-cells in the tumor to express the killer cell lectin-like receptor G1 (KLRG1). Tumors treated with combination antibody therapy showed 1.7-fold greater infiltration by these KLRG1+CD4+ effector T-cells than did those treated with α4-1BB alone.
Conclusions/significance: This study shows that combining T-cell co-inhibitory blockade with αCTLA-4 and active co-stimulation with α4-1BB promotes rejection of B16 melanoma in the context of a suitable vaccine. In addition, we identify KLRG1 as a useful marker for monitoring the anti-tumor immune response elicited by this therapy. These findings should aid in the design of future trials for the immunotherapy of melanoma.
Conflict of interest statement
Competing Interests: Dr. James P. Allison is a paid consultant for Bristol-Myers Squib and is the primary inventor on the patent “Blockade of T lymphocyte down-regulation associated with CTLA-4 signaling” (US Patent 7229628; Submitted 12/07/199; Published: 06/12/2007) which has been been licensed by UC Berkeley to Bristol-Myers Squib. This does not alter the authors' adherence to all the PLoS ONE policies on sharing data and materials.
Figures
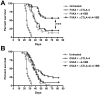
Figure 1. Anti-CTLA-4 and α4-1BB synergize in the context of FVAX.
Kaplan-Meier survival curves for mice challenged with 2.5×104 B16-BL6 cells and vaccinated on days 3, 6 and 9 with 1×106 of A) FVAX or B) GVAX intra-dermally and the indicated antibody or combination intra-peritoneally. Lack of survival was defined as death or tumor size >1000 mm3. Each curve represents 3 independent experiments of 10 mice per group. P values were calculated using the Log-rank (Mantel-Cox) test (* - p≤0.05, ** - p≤0.01, ***-p<0.001).
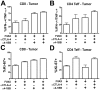
Figure 2. Combination αCTLA-4/α4-1BB therapy promotes high effector to regulatory T-cell ratios in the tumor.
Mice were challenged with 1.5×105 B16-BL6, treated with FVAX and the indicated antibody on days 3,6 and 9, and sacrificed on day 15. Percent of CD45+ tumor infiltrating lymphocytes of A) CD8+ T-cells, B) CD4+ effector (Teff) and C) regulatory (Treg) cells is shown. The ratios of D) CD8+ T-cells to Tregs and E) CD4+ Teff to Treg are shown. Values shown are for individually analyzed mice and are the sum of 5 independent experiments with 5–15 mice per group. The ratios of F) CD8+ T-cells to Tregs and G) CD4+ Teff to Treg are shown at Day 25 for individual mice from 2 independent experiments. Student's t-tests were performed to determine statistical significance between samples (* - p≤0.05, ** - p≤0.01, ***-p<0.001).
Figure 3. 4-1BB activation cooperates with CTLA-4 blockade to induce peripheral inflammatory cytokine production.
Mice challenged with 2.5×105 B16-OVA cells and treated on days 6, 9 and 12, were sacrificed on Day 14. T-cells were purified from tumor-draining (TDLN) and vaccine-draining (VDLN) lymph nodes, stained with antibodies, and sorted by flow cytometry into CD4+ and CD8+ subsets. Cytokine production was measured after 36 hours using the TH1/TH2/TH17 CBA Kit (BD) and is shown for A) 2×105 VDLN and B) 2×105 TDLN CD8 T-cells restimulated on 1×105 OVA 257–264 peptide pulsed DCs and for C) 2.5×105 CD4 T-cells restimulated on 1×105 OVA 323–339 peptide pulsed DCs. Data is shown for 3–4 independent experiments with 5–10 pooled mice per group.
Figure 4. Combination αCTLA-4/α4-1BB therapy enhances intra-tumoral T-cell cytokine production and proliferation.
Mice challenged with 2.5×105 B16-OVA cells and treated on days 6, 9 and 12, were sacrificed on Day 14. TIL were purified from 5–10 pooled tumors per group and enriched using the Miltenyi T-cell purification kit. 2×106 TIL were restimulated with 7.5×105 DC (1∶1 mix of MHC-I and MHC-II peptide pulsed DCs) for 8 hours in the presence of BD GolgiPlug. Cells were fixed using the FoxP3 kit and analyzed by flow cytometry for lymphocyte markers and intracellular IFN-γ and TNF-α production for A) CD8s and B) CD4 Teffs and also Ki67 expression for C) CD8s and D) CD4 Teffs. Data shown is from 4 independent experiments. All means shown are +/− S.E.M.
Figure 5. Combination αCTLA-4/α4-1BB therapy induces KLRG1 and PD-1 expression by tumor-infiltrating T-cells.
Mice challenged with 1.5×105 B16-BL6 cells and treated on days 3, 6 and 9, were sacrificed on Day 15. TIL were fixed and stained for lymphocyte lineage and activation markers using the FoxP3 fixation kit. Percent of A) CD8+ T-cells, B) CD4+ effector (Teff) and C) regulatory (Treg) cells expressing KLRG1, PD-1, and CTLA-4 are shown. The 4F10 clone of αCTLA-4 was used to detect cells on which CTLA-4 had been blocked in vivo using the 9D9 clone. Values shown are for individually analyzed mice and are the sum of 4–6 independent experiments with 5–15 mice per group. Student's t-tests were performed to determine statistical significance between samples(* - p≤0.05, ** - p≤0.01, ***-p<0.001).
Figure 6. High KLRG1 expression by tumor-infiltrating effector T-cells at Day-25 in mice treated with combination αCTLA-4/α4-1BB therapy.
Mice challenged with either 2.5×104 (Untreated and FVAX), 1.0×105 (FVAX+αCTLA-4 or α4-1BB), or 2.0×105 (FVAX+αCTLA-4/α4-1BB) B16-BL6 cells and treated on days 4, 7 and 10, and 13 were sacrificed on Day 25. TIL were fixed and stained for lymphocyte lineage and activation markers using the FoxP3 fixation kit. Percent of A) CD8+ T-cells, B) CD4+ effector (Teff) expressing KLRG1 are shown. Values shown are for individually analyzed mice and are the sum of 2 independent experiments with 10 mice per group. Student's t-tests were performed to determine statistical significance between samples(* - p≤0.05, ** - p≤0.01, ***-p<0.001).
Figure 7. Combination αCTLA-4/α4-1BB therapy induces higher tumor infiltration by CD4+KLRG1+ T-cells than α4-1BB alone.
Mice challenged with 1.5×105 B16-BL6 cells and treated on days 3, 6 and 9, were sacrificed on Day 15. The number of KLRG1+ T-cells of a given lineage was calculated by multiplying the %KLRG1+ TIL determined by flow cytometry by the measured number of CD45+ lymphocytes per mm3 of tumor. Data shown are ratios of the absolute number of KLRG1+ T-cells from combination treated tumors to the number of KLRG1+ T-cells from α4-1BB alone treated tumors. Ratios were calculated for 5 independent experiments with 5–15 mice per group.
Figure 8. Combination αCTLA-4/α4-1BB treatment generates more pro-inflammatory ratios of effector T-cells to MDSC in the context of FVAX versus GVAX.
Mice challenged with 1.5×105 B16-BL6 cells and treated on days 3, 6 and 9, were sacrificed on Day 15. TIL were fixed and stained for myeloid and lymphocyte lineage and activation markers using the FoxP3 fixation kit. The ratios of A) CD8+ T-cells to CD11b+GR-1+ MDSC and B) CD4+ Teff to MDSC are shown in the FVAX setting. Values shown are for individually analyzed mice and are the sum of 3 independent experiments with 5–15 mice per group. The ratios of C) CD8+ T-cells to MDSC and D) CD4+ effector T-cells to MDSC are shown in the context of GVAX for 10 individual mice per group. Student's t-tests were performed to determine statistical significance between samples (* - p≤0.05, ** - p≤0.01, ***-p<0.001).
Similar articles
- PD-1 and CTLA-4 combination blockade expands infiltrating T cells and reduces regulatory T and myeloid cells within B16 melanoma tumors.
Curran MA, Montalvo W, Yagita H, Allison JP. Curran MA, et al. Proc Natl Acad Sci U S A. 2010 Mar 2;107(9):4275-80. doi: 10.1073/pnas.0915174107. Epub 2010 Feb 16. Proc Natl Acad Sci U S A. 2010. PMID: 20160101 Free PMC article. - Focal radiation therapy combined with 4-1BB activation and CTLA-4 blockade yields long-term survival and a protective antigen-specific memory response in a murine glioma model.
Belcaid Z, Phallen JA, Zeng J, See AP, Mathios D, Gottschalk C, Nicholas S, Kellett M, Ruzevick J, Jackson C, Albesiano E, Durham NM, Ye X, Tran PT, Tyler B, Wong JW, Brem H, Pardoll DM, Drake CG, Lim M. Belcaid Z, et al. PLoS One. 2014 Jul 11;9(7):e101764. doi: 10.1371/journal.pone.0101764. eCollection 2014. PLoS One. 2014. PMID: 25013914 Free PMC article. - A comprehensive review on the role of co-signaling receptors and Treg homeostasis in autoimmunity and tumor immunity.
Kumar P, Bhattacharya P, Prabhakar BS. Kumar P, et al. J Autoimmun. 2018 Dec;95:77-99. doi: 10.1016/j.jaut.2018.08.007. Epub 2018 Aug 31. J Autoimmun. 2018. PMID: 30174217 Free PMC article. Review. - Immunotherapy of cancer with 4-1BB.
Vinay DS, Kwon BS. Vinay DS, et al. Mol Cancer Ther. 2012 May;11(5):1062-70. doi: 10.1158/1535-7163.MCT-11-0677. Epub 2012 Apr 24. Mol Cancer Ther. 2012. PMID: 22532596 Review.
Cited by
- Tumor necrosis factor superfamily signaling: life and death in cancer.
Ababneh O, Nishizaki D, Kato S, Kurzrock R. Ababneh O, et al. Cancer Metastasis Rev. 2024 Dec;43(4):1137-1163. doi: 10.1007/s10555-024-10206-6. Epub 2024 Oct 4. Cancer Metastasis Rev. 2024. PMID: 39363128 Free PMC article. Review. - The role of KLRG1: a novel biomarker and new therapeutic target.
Zhang Y, Chen S, Tang X, Peng Y, Jiang T, Zhang X, Li J, Liu Y, Yang Z. Zhang Y, et al. Cell Commun Signal. 2024 Jun 19;22(1):337. doi: 10.1186/s12964-024-01714-7. Cell Commun Signal. 2024. PMID: 38898461 Free PMC article. Review. - CAR-T cell therapy: a game-changer in cancer treatment and beyond.
Utkarsh K, Srivastava N, Kumar S, Khan A, Dagar G, Kumar M, Singh M, Haque S. Utkarsh K, et al. Clin Transl Oncol. 2024 Jun;26(6):1300-1318. doi: 10.1007/s12094-023-03368-2. Epub 2024 Jan 20. Clin Transl Oncol. 2024. PMID: 38244129 Review. - Targeted spatial proteomic analysis of CD8+ T- and myeloid cells in tonsillar cancer.
Altunbulakli C, Jimenez DG, Askmyr D, Sobti A, Swoboda S, Greiff L, Lindstedt M. Altunbulakli C, et al. Front Oncol. 2023 Nov 17;13:1253418. doi: 10.3389/fonc.2023.1253418. eCollection 2023. Front Oncol. 2023. PMID: 38044986 Free PMC article. - Intracellular Salmonella delivery of an exogenous immunization antigen refocuses CD8 T cells against cancer cells, eliminates pancreatic tumors and forms antitumor immunity.
Raman V, Howell LM, Bloom SMK, Hall CL, Wetherby VE, Minter LM, Kulkarni AA, Forbes NS. Raman V, et al. Front Immunol. 2023 Oct 5;14:1228532. doi: 10.3389/fimmu.2023.1228532. eCollection 2023. Front Immunol. 2023. PMID: 37868996 Free PMC article.
References
- Schneider H, Downey J, Smith A, Zinselmeyer BH, Rush C, et al. Reversal of the TCR stop signal by CTLA-4. Science. 2006;313:1972–1975. - PubMed
- Wing K, Onishi Y, Prieto-Martin P, Yamaguchi T, Miyara M, et al. CTLA-4 control over Foxp3+ regulatory T cell function. Science. 2008;322:271–275. - PubMed
- van Elsas A, Hurwitz AA, Allison JP. Combination immunotherapy of B16 melanoma using anti-cytotoxic T lymphocyte-associated antigen 4 (CTLA-4) and granulocyte/macrophage colony-stimulating factor (GM-CSF)-producing vaccines induces rejection of subcutaneous and metastatic tumors accompanied by autoimmune depigmentation. J Exp Med. 1999;190:355–366. - PMC - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous