α-catenin is a tumor suppressor that controls cell accumulation by regulating the localization and activity of the transcriptional coactivator Yap1 - PubMed (original) (raw)
α-catenin is a tumor suppressor that controls cell accumulation by regulating the localization and activity of the transcriptional coactivator Yap1
Mark R Silvis et al. Sci Signal. 2011.
Abstract
The Hippo pathway regulates contact inhibition of cell proliferation and, ultimately, organ size in diverse multicellular organisms. Inactivation of the Hippo pathway promotes nuclear localization of the transcriptional coactivator Yap1, a Hippo pathway effector, and can cause cancer. Here, we show that deletion of αE (α epithelial) catenin in the hair follicle stem cell compartment resulted in the development of skin squamous cell carcinoma in mice. Tumor formation was accelerated by simultaneous deletion of αE-catenin and the tumor suppressor-encoding gene p53. A small interfering RNA screen revealed a functional connection between αE-catenin and Yap1. By interacting with Yap1, αE-catenin promoted its cytoplasmic localization, and Yap1 showed constitutive nuclear localization in αE-catenin-null cells. We also found an inverse correlation between αE-catenin abundance and Yap1 activation in human squamous cell carcinoma tumors. These findings identify αE-catenin as a tumor suppressor that inhibits Yap1 activity and sequesters it in the cytoplasm.
Figures
Fig. 1. Conditional deletion of α E-catenin in hair follicles
(A) Model of growing hair follicle. Hair follicle stem cells localize to the bulge region. Matrix contains committed progenitors that differentiate and give rise to hair and inner root sheath. (B to D) Staining for LacZ activity in frozen sections from newborn P2, P6, and P30 GFAP-Cre/ROSA26Cre test mice. Red is nuclear fast red counterstain. (E) General appearance of 6 month-old αE-cateninfl/fl (Ctrl) and GFAP-Cre/αE-cateninfl/fl (α-cat cKO) mice. (F) Immunofluorescent staining of skin sections from P60 αE-cateninfl/fl (Ctrl) and GFAP-Cre/αE-cateninfl/fl (α-cat cKO) mice with anti-E-cadherin (red) and anti-α-catenin (green) antibodies. Note loss of α-catenin in hair follicles of α-cat cKO skin. (G) Immunofluorescent staining of skin sections from P35 αE-cateninfl/fl (Ctrl) and GFAP-Cre/αE-cateninfl/fl (α-cat cKO) mice with antibodies against Sox9, a stem and early progenitor marker (red) and α-catenin (green). α-catenin is absent in Sox9+ cells in α-cat cKO skin. Blue in F and G is nuclear DAPI stain. Scale bars: 70 μm in (B); 190 μm in (C) and (D); 47 μm in (F) and (G).
Fig. 2. αE-catenin is a tumor suppressor in skin keratoacanthoma
(A) General appearance of 2 month-old αE-cateninfl/fl (Ctrl) and tumor-bearing GFAP-Cre/αE-cateninfl/fl (α-cat cKO) mice. (B) Kaplan-Meier survival curves of αE-cateninfl/fl (Ctrl) (N=19) and GFAP-Cre/αE-cateninfl/fl (α-cat cKO) (N=59) mice. Statistical significance was determined by the logrank test (P=<0.0001). (C to H) Hematoxylin and eosin staining of skin sections from 2 month-old αE-cateninfl/fl (Ctrl) and tumor-bearing GFAP-Cre/αE-cateninfl/fl (α-cat cKO) mice. Images in E to H show higher magnifications of the sections shown in C and D. (I to P) Immunofluorescent staining of skin sections from 2 month-old αE-cateninfl/fl (Ctrl) and tumor-bearing GFAP-Cre/αE-cateninfl/fl (α-cat cKO) mice with anti-BrdU (red), anti-keratin 5 [green in (I) to (L)], anti-keratin 6 [green in (M) and (N)], anti-involucrin [green in (O) and (P)] antibodies. Blue in I to P is nuclear DAPI stain. Scale bars: 500 μm in (C) and (D); 214 μm in (E) and (F); 53 μm in (G) and (H); 75 μm in (I) and (J); 19 μm in (K) and (L); 75 μm in (M) to (P).
Fig. 3. Early onset keratoacanthoma tumors in GFAP-Cre/αE-cateninfl/fl/p53fl/fl mice and loss of αE-catenin abundance in human keratoacanthomas
(A) Western blot analysis of total protein extracts from skins of αE-cateninfl/fl (Ctrl) and GFAP-Cre/αE-cateninfl/fl mice with anti-p53, anti-Arfp16, and anti-β-tubulin antibodies. N=3. (B) Kaplan-Meier survival curves for GFAP-Cre/αE-cateninfl/fl/p53fl/fl (N=30), GFAP-Cre/p53fl/fl (N=19), and wild-type (Ctrl) (N=19) mice. Curves for GFAP-Cre/αE-cateninfl/f/p53fl/fl and GFAP-Cre/p53fl/fl mice are different P<0.0001 (logrank test). Note: Due GFAP-Cre activity in brain, GFAP-Cre/p53fl/fl mice die from brain tumors; however, they do not have a skin phenotype at the time of euthanasia. (C) General appearance of 4 month-old GFAP-Cre/p53fl/fl (p53 cKO) F) and GFAP-Cre/αE-cateninfl/fl/p53fl/fl (α-cat+p53 cKO) mice. (D to Hematoxylin and eosin staining of skin tumor from 4 month-old GFAP-Cre/αE-cateninfl/fl/p53fl/fl (α-cat+p53 cKO) mouse. Images in E and F show higher magnifications of the section shown in D. (G to L) Immunofluorescent staining of human keratoacanthoma tumor with anti-α-catenin (red) and anti-pan-cytokeratin (green) antibodies. α-catenin is present in normal areas and not detectable in tumor cells. Blue in I to L is nuclear DAPI stain. Scale bars: 1 mm in (D); 0.4 mm in (E); 100 μm in (F); 30 μm in (G) to (I); 7.5 μm in (J) to (L).
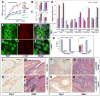
Fig. 4. Yap1 is necessary for α-catenin-mediated loss of contact inhibition and hyperplasia
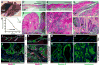
(A) Growth curves of αE-cateninfl/fl (Ctrl) and αE-catenin−/− (α-cat−/−) cells expressing vector or full-length αE-catenin (Ctrl+α-cat, α-cat−/−+α-cat). Note prominent inhibition of cell accumulation in confluent Ctrl keratinocytes and continued accumulation of α-cat−/− keratinocytes. The phenotype is rescued by re-expression of full-length αE-catenin. (B) αE-cateninfl/fl or αE-catenin−/− keratinocytes were plated at high density in siRNA-Lipofectamine mixture in triplicate and cultured for 5 days. Cell numbers at the end of culture were determined by MTT assay. Under these conditions, αE-catenin−/− cells were not contact inhibited and show increased cell accumulation. This phenotype was replicated in αE-cateninfl/fl cells by transfection of siRNAs targeting αE-catenin. Bar graph shows mean values ± SD. N=3. **, P<0.01 by t-test. **(C)** Yap1 is necessary for the loss of contact inhibition in αE-catenin-null cells. Validation of the functional connection of Yap1 to α-catenin previously identified by siRNA screen (fig. S10), using siRNA oligos targeting disparate regions of Yap1. Western control is shown in fig. S11. Bar graph shows mean values ± SD. N=3. **(D)** Validation of the specificity of Yap1 siRNA hit using re-expression of human Yap2, which is not targeted by anti-mouse Yap1 siRNAs. **(E** to **J)** Representative images of confluent _αE-cateninfl/fl_ (Ctrl) and _αE-catenin−/−_ (_α-cat−/−_) keratinocytes stained with anti-Yap1 and anti-α-catenin antibodies. **(K)** Quantification of Yap1 localization illustrated in (E) to (J) and fig. S12. Bar graph shows mean values ± SD. Number of cells counted was >50 per condition. P value was determined by Mann-Whitney test. (L to O) Hematoxylin and eosin (H&E) and anti-Yap1 immunohistochemical staining of skin sections from 8 month-old αE-cateninfl/fl (Ctrl) and GFAP-Cre/αE-cateninfl/fl (α-cat cKO) mice. (P to S) H&E and anti-Yap1 immunohistochemical staining of keratoacanthoma tumors from GFAP-Cre/αE-cateninfl/fl (α-cat cKO), GFAP-Cre/αE-cateninfl/fl/p53fl/fl (α-cat+p53 cKO) mice. Blue in G and J is nuclear DAPI stain. Scale bars: 21 μm in (E) to (J); 35 μm in (L) to (O); 50 μm in (P) to (S).
Fig. 5. αE-catenin interacts with Yap1 and inhibits its nuclear localization and transcriptional activity
(A to H) H&E and anti-Yap1 immunohistochemical staining of human keratoacanthoma tumors with high and low abundance of α-catenin. (I) Box and whisker plot showing quantification of differences in Yap1 abundance in keratoacanthoma tumors with low (N=5) and high (N=5) α-catenin abundance. Cells were counted in 3 randomly selected images per tumor. Number of cells counted per each image was >170. The plots show percentage of tumor cells displaying nuclear staining for Yap1. Lines within boxes, median values. Upper and lower borders of the boxes, 25th and 75th percentiles. Upper and lower bars, maximum and minimum values. Statistical significance was assessed by the Mann-Whitney test. Scale bars: 155 μm in (A) and (B), (E) and (F); 50 μm in (C) and (D), (G) and (H). (J) Coimmunoprecipitation of endogenous α-catenin and Yap1. Proteins were extracted from cultured keratinocytes (IN) and immunoprecipitated (IP) with IgG controls, anti-Yap1, or anti-α-catenin antibodies and analyzed by Western blotting (WB) with anti-α-catenin or anti-Yap1 antibodies. (K and L) αE-catenin inhibits Yap1 transcriptional activity. Indicated constructs with or without siRNAs were cotransfected with a Gal4-TEAD firefly luciferase reporter and a Renilla luciferase control plasmid into HEK 293FT cells for gain-of-function experiments (K) or wild-type mouse keratinocytes for loss-of-function experiments (L). Reporter luciferase activity was normalized to Renilla luciferase activity. Bar graph shows mean values ± SE. Statistical significance was determined by ANOVA test. (M) Model showing the role of α-catenin in regulating Yap1 localization and transcriptional activity.
Similar articles
- αE-catenin inhibits a Src-YAP1 oncogenic module that couples tyrosine kinases and the effector of Hippo signaling pathway.
Li P, Silvis MR, Honaker Y, Lien WH, Arron ST, Vasioukhin V. Li P, et al. Genes Dev. 2016 Apr 1;30(7):798-811. doi: 10.1101/gad.274951.115. Epub 2016 Mar 24. Genes Dev. 2016. PMID: 27013234 Free PMC article. - Dishevelled has a YAP nuclear export function in a tumor suppressor context-dependent manner.
Lee Y, Kim NH, Cho ES, Yang JH, Cha YH, Kang HE, Yun JS, Cho SB, Lee SH, Paclikova P, Radaszkiewicz TW, Bryja V, Kang CG, Yuk YS, Cha SY, Kim SY, Kim HS, Yook JI. Lee Y, et al. Nat Commun. 2018 Jun 12;9(1):2301. doi: 10.1038/s41467-018-04757-w. Nat Commun. 2018. PMID: 29895829 Free PMC article. - Yap1 acts downstream of α-catenin to control epidermal proliferation.
Schlegelmilch K, Mohseni M, Kirak O, Pruszak J, Rodriguez JR, Zhou D, Kreger BT, Vasioukhin V, Avruch J, Brummelkamp TR, Camargo FD. Schlegelmilch K, et al. Cell. 2011 Mar 4;144(5):782-95. doi: 10.1016/j.cell.2011.02.031. Cell. 2011. PMID: 21376238 Free PMC article. - Mst1 and Mst2 protein kinases restrain intestinal stem cell proliferation and colonic tumorigenesis by inhibition of Yes-associated protein (Yap) overabundance.
Zhou D, Zhang Y, Wu H, Barry E, Yin Y, Lawrence E, Dawson D, Willis JE, Markowitz SD, Camargo FD, Avruch J. Zhou D, et al. Proc Natl Acad Sci U S A. 2011 Dec 6;108(49):E1312-20. doi: 10.1073/pnas.1110428108. Epub 2011 Oct 31. Proc Natl Acad Sci U S A. 2011. PMID: 22042863 Free PMC article. - The role of Hippo-YAP signaling in squamous cell carcinomas.
Maehama T, Nishio M, Otani J, Mak TW, Suzuki A. Maehama T, et al. Cancer Sci. 2021 Jan;112(1):51-60. doi: 10.1111/cas.14725. Epub 2020 Dec 9. Cancer Sci. 2021. PMID: 33159406 Free PMC article. Review.
Cited by
- The Hippo pathway target, YAP, promotes metastasis through its TEAD-interaction domain.
Lamar JM, Stern P, Liu H, Schindler JW, Jiang ZG, Hynes RO. Lamar JM, et al. Proc Natl Acad Sci U S A. 2012 Sep 11;109(37):E2441-50. doi: 10.1073/pnas.1212021109. Epub 2012 Aug 13. Proc Natl Acad Sci U S A. 2012. PMID: 22891335 Free PMC article. - Targeting the Hippo Signaling Pathway for Tissue Regeneration and Cancer Therapy.
Juan WC, Hong W. Juan WC, et al. Genes (Basel). 2016 Aug 30;7(9):55. doi: 10.3390/genes7090055. Genes (Basel). 2016. PMID: 27589805 Free PMC article. Review. - MAGI1 inhibits the AMOTL2/p38 stress pathway and prevents luminal breast tumorigenesis.
Kantar D, Mur EB, Mancini M, Slaninova V, Salah YB, Costa L, Forest E, Lassus P, Géminard C, Boissière-Michot F, Orsetti B, Theillet C, Colinge J, Benistant C, Maraver A, Heron-Milhavet L, Djiane A. Kantar D, et al. Sci Rep. 2021 Mar 11;11(1):5752. doi: 10.1038/s41598-021-85056-1. Sci Rep. 2021. PMID: 33707576 Free PMC article. - A feedback loop between plakophilin 4 and YAP signaling regulates keratinocyte differentiation.
Müller L, Gutschner T, Hatzfeld M. Müller L, et al. iScience. 2024 Aug 19;27(9):110762. doi: 10.1016/j.isci.2024.110762. eCollection 2024 Sep 20. iScience. 2024. PMID: 39286493 Free PMC article. - Targeting the Hippo/YAP/TAZ signalling pathway: Novel opportunities for therapeutic interventions into skin cancers.
Howard A, Bojko J, Flynn B, Bowen S, Jungwirth U, Walko G. Howard A, et al. Exp Dermatol. 2022 Oct;31(10):1477-1499. doi: 10.1111/exd.14655. Epub 2022 Aug 12. Exp Dermatol. 2022. PMID: 35913427 Free PMC article. Review.
References
- Lien WH, Klezovitch O, Vasioukhin V. Cadherin-catenin proteins in vertebrate development. CurrOpin Cell Biol. 2006;18:499–506. - PubMed
- Clevers H. Wnt/beta-catenin signaling in development and disease. Cell. 2006;127:469–480. - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials
Miscellaneous