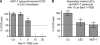NF-κB mediates the 12(S)-HETE-induced endothelial to mesenchymal transition of lymphendothelial cells during the intravasation of breast carcinoma cells - PubMed (original) (raw)
. 2011 Jul 12;105(2):263-71.
doi: 10.1038/bjc.2011.194. Epub 2011 May 31.
K Viola, B Giessrigl, N Huttary, I Raab, R Kalt, S Krieger, T P N Vo, S Madlener, S Bauer, B Marian, M Hämmerle, N Kretschy, M Teichmann, B Hantusch, S Stary, C Unger, M Seelinger, A Eger, R Mader, W Jäger, W Schmidt, M Grusch, H Dolznig, W Mikulits, G Krupitza
Affiliations
- PMID: 21629247
- PMCID: PMC3142797
- DOI: 10.1038/bjc.2011.194
NF-κB mediates the 12(S)-HETE-induced endothelial to mesenchymal transition of lymphendothelial cells during the intravasation of breast carcinoma cells
C Vonach et al. Br J Cancer. 2011.
Abstract
Background: The intravasation of breast cancer into the lymphendothelium is an early step of metastasis. Little is known about the mechanisms of bulky cancer invasion into lymph ducts.
Methods: To particularly address this issue, we developed a 3-dimensional co-culture model involving MCF-7 breast cancer cell spheroids and telomerase-immortalised human lymphendothelial cell (LEC) monolayers, which resembles intravasation in vivo and correlated the malignant phenotype with specific protein expression of LECs.
Results: We show that tumour spheroids generate 'circular chemorepellent-induced defects' (CCID) in LEC monolayers through retraction of LECs, which was induced by 12(S)-hydroxyeicosatetraenoic acid (HETE) secreted by MCF-7 spheroids. This 12(S)-HETE-regulated retraction of LECs during intravasation particularly allowed us to investigate the key regulators involved in the motility and plasticity of LECs. In all, 12(S)-HETE induced pro-metastatic protein expression patterns and showed NF-κB-dependent up-regulation of the mesenchymal marker protein S100A4 and of transcriptional repressor ZEB1 concomittant with down-regulation of the endothelial adherence junction component VE-cadherin. This was in accordance with ∼50% attenuation of CCID formation by treatment of cells with 10 μM Bay11-7082. Notably, 12(S)-HETE-induced VE-cadherin repression was regulated by either NF-κB or by ZEB1 since ZEB1 siRNA knockdown abrogated not only 12(S)-HETE-mediated VE-cadherin repression but inhibited VE-cadherin expression in general.
Interpretation: These data suggest an endothelial to mesenchymal transition-like process of LECs, which induces single cell motility during endothelial transmigration of breast carcinoma cells. In conclusion, this study demonstrates that the 12(S)-HETE-induced intravasation of MCF-7 spheroids through LECs require an NF-κB-dependent process of LECs triggering the disintegration of cell-cell contacts, migration, and the generation of CCID.
Figures
Figure 1
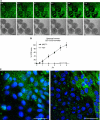
CCID formation by cell migration. (A) Time lap experiment show the same microscopic power field after 0–5 h co-culture of LECs (upper panel; cytotracker green, FITC filter) and MCF-7 spheroids (lower panel; phase contrast); The images show the progression of CCID formation over time. No apoptotic features were observed. Scale bars: 200 _μ_m. (B) The gradual increase of CCID areas over time was measured underneath five MCF-7 spheroids or human normal lung fibroblast spheroids (HLF) after the indicated time points using Axiovision software (Zeiss). Error bars indicate s.e.m. (C) LECs were grown on coverslips until confluence when MCF-7 spheroids were transferred on top of LECs and co-incubated for 4 h at 37°C to allow CCID formation. LECs were stained with respective antibodies. Confocal laser scanning microscopy of immunocytochemically stained LECs at the rim of CCID (upper right diagon each, which was the part covered by the MCF-7 spheroid) show elevated levels of phosphorylation (green; FITC filter) of MYPT threonine-696 (left panel) and MLC2 serine-19 (right panel), indicating increased cell mobility. Nuclei are stained with DAPI (blue). Scale bars: 45 _μ_m.
Figure 2
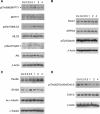
Modulation of protein expression and posttranslational modifications in LECs. LEC monolayers were incubated with 1 _μ_M synthetic 12(S)-HETE and analysed by western blotting after 0.2, 0.5, 1.0, 2.0, and 4.0 h. Equal sample loading was controlled by Ponceau S staining, _β_-actin (A, B, D), or _α_-tubulin (C) expression. Co, untreated LECs.
Figure 3
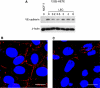
Analysis of VE-cadherin expression in LECs. (A) LECs were treated with 1 μ
M
12(S)-HETE for 0.2, 0.5, 2, 4, and 8 h. Then, cells were harvested and protein lysates were analysed by western blotting. MCF-7 cells were used as negative control. Equal sample loading was controlled by Ponceau S staining and _β_-actin analysis. Confocal immunofluorescence images of LECs next to a spheroid (B) and underneath an MCF-7 spheroid (C). LECs were grown on coverslips until confluence when MCF-7 spheroids were transferred on top of LECs and co-incubated for 4 h at 37°C to allow CCID formation. LECs were stained with anti-VE-cadherin antibody (red) and DAPI (blue). (B) Distant to a spheroid, VE-cadherin structures appear well developed, whereas (C) VE-cadherin interactions are disrupted underneath an MCF-7 spheroid. Scale bar: 15 μ
M
. The colour reproduction of this figure is available at the British Journal of Cancer journal online.
Figure 4
Effect of ZEB1 suppression on VE-cadherin regulation by 12(S)-HETE. LECs were transiently transfected with two different siRNAs against ZEB1 (+: siRNA1; +′: siRNA2), or with scrambled siRNA. LECs were subsequently treated with 1 μ
M
12(S)-HETE and analysed by western blotting using antibodies against ZEB1 and VE-cadherin. Equal sample loading was controlled by _β_-actin expression.
Figure 5
Quantitative analysis of formation and inhibition of CCID in LEC monolayers by MCF-7 spheroids formation. LECs were seeded into 24-well plates and allowed to grow for 2 days until confluence when LECs were stained with cytotracker green. (A) MCF-7spheroids, which were treated with different concentrations (solvent, 1, 5, 10, 15, and 25 μ
M
) of Bay11-7082 for 0.5 h at 37°C, were transferred on top of LECs. (B) Either LECs or MCF-7 spheroids were treated with the Bay11-7082 for 0.5 h, which was entirely washed off before both cell types were co-cultivated. The 3D-MCF-7 spheroids/LEC monolayer co-cultures were incubated for 4 h at 37°C. The size of CCIDs, which were formed by MCF-7 spheroids in the LEC monolayer in this time period, was measured using a Zeiss Axiovert microscope and Axiovision software. In the solvent treated controls, the CCID sizes in LEC monolayers were set 100%. For each condition, the gap area of at least 12 spheroids was measured. Error bars indicate standard error of the mean. Asterisks show significant differences in the inhibition of CCID formation compared with control (*P<0.05).
Figure 6
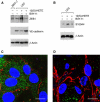
Analysis of mesenchymal marker expression in LECs after intervention with NF-_κ_B signalling. LECs were pretreated with 10 μ
M
of the I-κ_B_α phosphorylation inhibitor Bay11-7082 for 0.5 h and then stimulated with 1 μ
M
12(S)-HETE for 0.2 h. Cells were harvested and analysed by western blotting using (A) anti-ZEB1 and anti-VE-cadherin antibodies. MCF-7 cells were used as negative control. (B) Blots were analysed with anti-S100A4 antibody. Equal sample loading was controlled by Ponceau S staining and _β_-actin analysis. Confocal immunofluorescence images of LECs at the rim of CCID (C) induced by an MCF-7 spheroid; (D) and from a similar position after treatment with 10 μ
M
Bay11-7082. LECs were grown on coverslips until confluence when MCF-7 spheroids were transferred on top of LECs and co-incubated for 4 h at 37°C to allow CCID formation. LECs were stained with anti-S400A4 antibody (green), anti-VE-cadherin antibody (red), and DAPI (blue). (C) S100A4 is well expressed and VE-cadherin interactions are disrupted. (D) Upon Bay11-7082 treatment, VE-cadherin structures again appear well developed (although unconnected to VE-cadherin structures of neighbouring cells), whereas S100A4 expression is decreased. Scale bar: 15 μ
M
.
Similar articles
- Bay11-7082 inhibits the disintegration of the lymphendothelial barrier triggered by MCF-7 breast cancer spheroids; the role of ICAM-1 and adhesion.
Viola K, Kopf S, Huttary N, Vonach C, Kretschy N, Teichmann M, Giessrigl B, Raab I, Stary S, Krieger S, Keller T, Bauer S, Hantusch B, Szekeres T, de Martin R, Jäger W, Mikulits W, Dolznig H, Krupitza G, Grusch M. Viola K, et al. Br J Cancer. 2013 Feb 19;108(3):564-9. doi: 10.1038/bjc.2012.485. Epub 2012 Oct 23. Br J Cancer. 2013. PMID: 23093227 Free PMC article. - In vitro inhibition of breast cancer spheroid-induced lymphendothelial defects resembling intravasation into the lymphatic vasculature by acetohexamide, isoxsuprine, nifedipin and proadifen.
Kretschy N, Teichmann M, Kopf S, Atanasov AG, Saiko P, Vonach C, Viola K, Giessrigl B, Huttary N, Raab I, Krieger S, Jäger W, Szekeres T, Nijman SM, Mikulits W, Dirsch VM, Dolznig H, Grusch M, Krupitza G. Kretschy N, et al. Br J Cancer. 2013 Feb 19;108(3):570-8. doi: 10.1038/bjc.2012.580. Epub 2013 Jan 8. Br J Cancer. 2013. PMID: 23299527 Free PMC article. - Xanthohumol attenuates tumour cell-mediated breaching of the lymphendothelial barrier and prevents intravasation and metastasis.
Viola K, Kopf S, Rarova L, Jarukamjorn K, Kretschy N, Teichmann M, Vonach C, Atanasov AG, Giessrigl B, Huttary N, Raab I, Krieger S, Strnad M, de Martin R, Saiko P, Szekeres T, Knasmüller S, Dirsch VM, Jäger W, Grusch M, Dolznig H, Mikulits W, Krupitza G. Viola K, et al. Arch Toxicol. 2013 Jul;87(7):1301-12. doi: 10.1007/s00204-013-1028-2. Epub 2013 Mar 17. Arch Toxicol. 2013. PMID: 23503627 - Inhibition of tumour spheroid-induced prometastatic intravasation gates in the lymph endothelial cell barrier by carbamazepine: drug testing in a 3D model.
Teichmann M, Kretschy N, Kopf S, Jarukamjorn K, Atanasov AG, Viola K, Giessrigl B, Saiko P, Szekeres T, Mikulits W, Dirsch VM, Huttary N, Krieger S, Jäger W, Grusch M, Dolznig H, Krupitza G. Teichmann M, et al. Arch Toxicol. 2014 Mar;88(3):691-9. doi: 10.1007/s00204-013-1183-5. Epub 2013 Dec 19. Arch Toxicol. 2014. PMID: 24352538 - In vitro characterisation of the anti-intravasative properties of the marine product heteronemin.
Kopf S, Viola K, Atanasov AG, Jarukamjorn K, Rarova L, Kretschy N, Teichmann M, Vonach C, Saiko P, Giessrigl B, Huttary N, Raab I, Krieger S, Schumacher M, Diederich M, Strnad M, de Martin R, Szekeres T, Jäger W, Dirsch VM, Mikulits W, Grusch M, Dolznig H, Krupitza G. Kopf S, et al. Arch Toxicol. 2013 Oct;87(10):1851-61. doi: 10.1007/s00204-013-1045-1. Epub 2013 Mar 30. Arch Toxicol. 2013. PMID: 23543012
Cited by
- Evaluation of the Metabolite Profile of Fish Oil Omega-3 Fatty Acids (n-3 FAs) in Micellar and Enteric-Coated Forms-A Randomized, Cross-Over Human Study.
Ibi A, Chang C, Kuo YC, Zhang Y, Du M, Roh YS, Gahler R, Hardy M, Solnier J. Ibi A, et al. Metabolites. 2024 May 7;14(5):265. doi: 10.3390/metabo14050265. Metabolites. 2024. PMID: 38786742 Free PMC article. - GTF2H4 regulates partial EndMT via NF-κB activation through NCOA3 phosphorylation in ischemic diseases.
Fang Z, Zhao G, Zhao S, Yu X, Feng R, Zhang YE, Li H, Huang L, Guo Z, Zhang Z, Abdurahman M, Hong H, Li P, Wu B, Zhu J, Zhong X, Huang D, Lu H, Zhao X, Chen Z, Zhang W, Guo J, Zheng H, He Y, Qin S, Lu H, Zhao Y, Wang X, Ge J, Li H. Fang Z, et al. Innovation (Camb). 2024 Jan 8;5(2):100565. doi: 10.1016/j.xinn.2024.100565. eCollection 2024 Mar 4. Innovation (Camb). 2024. PMID: 38379791 Free PMC article. - YB-1 regulates mesothelioma cell migration via snail but not EGFR, MMP1, EPHA5 or PARK2.
Schelch K, Eder S, Zitta B, Phimmachanh M, Johnson TG, Emminger D, Wenninger-Weinzierl A, Sturtzel C, Poplimont H, Ries A, Hoetzenecker K, Hoda MA, Berger W, Distel M, Dome B, Reid G, Grusch M. Schelch K, et al. Mol Oncol. 2024 Apr;18(4):815-831. doi: 10.1002/1878-0261.13367. Epub 2023 Jan 25. Mol Oncol. 2024. PMID: 36550787 Free PMC article. - Role of Lymphatic Endothelium in Vascular Escape of Engineered Human Breast Microtumors.
Seibel AJ, Kelly OM, Dance YW, Nelson CM, Tien J. Seibel AJ, et al. Cell Mol Bioeng. 2022 Nov 7;15(6):553-569. doi: 10.1007/s12195-022-00745-9. eCollection 2022 Dec. Cell Mol Bioeng. 2022. PMID: 36531861 Free PMC article. - Impact of Non-Coding RNAs on Chemotherapeutic Resistance in Oral Cancer.
Yamaguchi K, Yamamoto T, Chikuda J, Shirota T, Yamamoto Y. Yamaguchi K, et al. Biomolecules. 2022 Feb 9;12(2):284. doi: 10.3390/biom12020284. Biomolecules. 2022. PMID: 35204785 Free PMC article. Review.
References
- Ambartsumian N, Klingelhofer J, Grigorian M, Christensen C, Kriajevska M, Tulchinsky E, Georgiev G, Berezin V, Bock E, Rygaard J, Cao R, Cao Y, Lukanidin E (2001) The metastasis-associated Mts1(S100A4) protein could act as an angiogenic factor. Oncogene 20: 4685–4695 - PubMed
- Birukova AA, Smurova K, Birukov KG, Kaibuchi K, Garcia JG, Verin AD (2004a) Role of Rho GTPases in thrombin-induced lung vascular endothelial cells barrier dysfunction. Microvasc Res 67: 64–77 - PubMed
- Birukova AA, Smurova K, Birukov KG, Usatyuk P, Liu F, Kaibuchi K, Ricks-Cord A, Natarajan V, Alieva I, Garcia JG, Verin AD (2004b) Microtubule disassembly induces cytoskeletal remodeling and lung vascular barrier dysfunction: role of Rho-dependent mechanisms. J Cell Physiol 201: 55–70 - PubMed
- Boye K, Grotterod I, Aasheim HC, Hovig E, Maelandsmo GM (2008) Activation of NF-kappaB by extracellular S100A4: analysis of signal transduction mechansims and identification of target genes. Int J Cancer 123/6(p4): 1301–1310 - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Research Materials