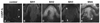Keratinocyte expression of calcitonin gene-related peptide β: implications for neuropathic and inflammatory pain mechanisms - PubMed (original) (raw)
Comparative Study
. 2011 Sep;152(9):2036-2051.
doi: 10.1016/j.pain.2011.04.033. Epub 2011 Jun 17.
Travis Barr, Lucy Gee, Jeff Vickers, James Wymer, Elisa Borsani, Luigi Rodella, Spiro Getsios, Trisha Burdo, Elan Eisenberg, Udayan Guha, Robert Lavker, John Kessler, Sridar Chittur, Dennis Fiorino, Frank Rice, Phillip Albrecht
Affiliations
- PMID: 21641113
- PMCID: PMC3157543
- DOI: 10.1016/j.pain.2011.04.033
Comparative Study
Keratinocyte expression of calcitonin gene-related peptide β: implications for neuropathic and inflammatory pain mechanisms
Quanzhi Hou et al. Pain. 2011 Sep.
Abstract
Calcitonin gene-related peptide (CGRP) is a vasodilatory peptide that has been detected at high levels in the skin, blood, and cerebrospinal fluid (CSF) under a variety of inflammatory and chronic pain conditions, presumably derived from peptidergic C and Aδ innervation. Herein, CGRP immunolabeling (IL) was detected in epidermal keratinocytes at levels that were especially high and widespread in the skin of humans from locations afflicted with postherpetic neuralgia (PHN) and complex region pain syndrome type 1 (CRPS), of monkeys infected with simian immunodeficiency virus, and of rats subjected to L5/L6 spinal nerve ligation, sciatic nerve chronic constriction, and subcutaneous injection of complete Freund's adjuvant. Increased CGRP-IL was also detected in epidermal keratinocytes of transgenic mice with keratin-14 promoter driven overexpression of noggin, an antagonist to BMP-4 signaling. Transcriptome microarray, quantitative Polymerase Chain Reaction (qPCR), and Western blot analyses using laser-captured mouse epidermis from transgenics, monolayer cultures of human and mouse keratinocytes, and multilayer human keratinocyte organotypic cultures, revealed that keratinocytes express predominantly the beta isoform of CGRP. Cutaneous peptidergic innervation has been shown to express predominantly the alpha isoform of CGRP. Keratinocytes also express the cognate CGRP receptor components, Calcitonin receptor-like receptor (CRLR), Receptor activity-modifying protein 1 (RAMP1), CGRP-receptor component protein (RCP) consistent with known observations that CGRP promotes several functional changes in keratinocytes, including proliferation and cytokine production. Our results indicate that keratinocyte-derived CGRPβ may modulate epidermal homeostasis through autocrine/paracrine signaling and may contribute to chronic pain under pathological conditions.
Copyright © 2011 International Association for the Study of Pain. All rights reserved.
Conflict of interest statement
The authors declare that there are no real or percieved conflicts of interest associated with the research data presented.
Figures
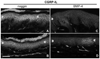
Figure 1. Increased CGRP expression in keratinocytes from human painful conditions
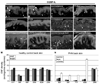
A–H) Images from sections of thoracic back skin biopsies processed with polyclonal anti-human CGRPα (A–F) captured at the same camera settings, and monoclonal anti-human CGRPα (G,H) captured at a higher camera sensitivity. Keratinocyte labeling is shown between arrows. Innervation labeling is shown with arrowheads. A, B) Examples of the variations in CGRP-IL expression among keratinocytes as shown in images from the left and right side respectively in a control subject (23-year old male) that had the most intense expression encountered among six control subjects. The CGRP-IL is heterogenous among keratinocytes mostly located in the middle third of the epidermis. C, D) Examples of CGRP-IL labeling among keratinocytes in a subject (29-year-old male) that had the least difference in labeling intensity between the PHN afflicted skin (D, VAS = 6.5) and the nonpainful unafflicted mirror-image contralateral skin (C). E, F) Examples of CGRP-IL labeling in a subject (48 year-old male, VAS = 5.0) that was representative of most of the PHN afflicted skin (F) and nonpainful unafflicted mirror-image contralateral skin (E). As revealed with the polyclonal antibody, nonpainful contralateral skin consistently had less intense and more heterogenous labeling of keratinocytes, whereas PHN skin had more intense and more homogenous labeling. In both the afflicted and unafflicted skin of the PHN subjects, CGRP-IL was expressed among keratinocytes spanning most of the thickness of the epidermis. In the PHN skin, the stratum basalis was usually less intensely labeled than the more superficial strata. CGRP positive innervation (arrowheads) appears to be less in PHN skin than in contralateral skin or control skin. E–H) Comparison of polyclonal (E, F) versus monoclonal (G, H) CGRP-IL in sections from the same biopsies from the same subject. Monoclonal CGRP-IL of keratinocytes was far less intense and more heterogenous than polyclonal labeling, but was still more intense and widespread among keratinocytes in the PHN (H) versus the contralateral skin (G). Because of the higher camera sensitivity setting, innervation labeled with the monoclonal antibody appears more intense than that with the polyclonal antibody (arrowheads). I–L) Examples of polyclonal CGRP-IL among keratinocytes from the hypothenar (I, J) and lateral foot (K, L) of control subjects (I, K) and subjects with severe CRPS type 1 (J, L). CGRP-IL among keratinocytes has a very low intensity in control skin and a moderate to high intensity among CRPS skin. In the CRPS skin, there are fewer and more weakly CGRP-IL detectable axons and endings in the upper dermis and epidermis than in control skin (arrowheads). Scale bar = 65µm in A–H, 50µm in I–L. M, N) Similar to other published reports, PGP-IL of epidermal endings from healthy controls (M) and the PHN patient cohort (N) reveals decreased epidermal nerve fiber density among the active PHN and ipsilateral sites compared with a mirror-image contralateral site, in 4 of the 5 PHN subjects, whereas healthy controls showed no lateral differences.
Figure 2. Nerve injury models of neuropathic pain increase CGRP expression in epidermal keratinocytes
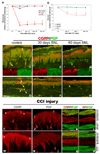
Nocicieptive behaviors were induced in rats by SNL (A) or sciatic CCI (B), and following SNL (C–H) or sciatic CCI (I–P), CGRP-IL increases are evident in hindpaw epidermal keratinocytes (red chevrons), with a reduction in peptidergic cutaneous innervation compared to control skin (yellow and red arrows). A) Graph depicting the extent of chronic neuropathic mechanical allodynia (Von Frey filaments) following SNL, demonstrating persistence out to at least 60 days, at a time corresponding to increased CGRP-IL among the keratinocytes. B) Graph depicting the extent of chronic neuropathic thermal hyperalgesia (Hargreaves test) following CCI, demonstrating persistence out to 14 days, at a time corresponding to increased CGRP-IL among the keratinocytes. C–P) Representative photomicrographs of single or double (merged) label immunocytochemistry for calcitonin-gene related peptide (CGRP, red) or 200kD neurofilament (NF, red) and protein gene product 9.5 (PGP, green). Previous studies have shown that anti-PGP labels all types of innervation, whereas 200kD NF immunolabeling is normally expressed in, and limited to, thick caliber Aβ fibers and thin caliber Aδ fibers. C fibers normally lack NF-IL. CGRP-IL is normally observed in subsets of C and Aδ fibers. C,D) Control, unoperated rats show intense CGRP-IL of axonal innervation (PGP positive, double labeled structures) among the epidermal endings, dermal vasculature endings, and subepidermal and dermal fibers (yellow arrows). Epidermal keratinocyte CGRP-IL (red chevrons) is heterogeneous and moderate among the volar pad crown (C) and flat (D) glabrous hindpaw skin, and remains largely suprabasal. E,F) By 30 days following SNL, a near complete loss of neural innervation is observed (i.e., no PGP nerve labeling; small green arrow in F shows a Langerhans cell, which are known to express PGP), while epidermal keratinocyte CGRP expression appears more extensive throughout suprabasal keratinocytes among the pad crown (E) and flat (F) glabrous hindpaw skin (red chevrons). G, H) By 60 days following SNL, neural innervation has begun to return (PGP axons, green arrows), but largely without a CGRP contingent, whereas the epidermal keratinocyte expression of CGRP remains elevated among pad crown (G) and flat (H) glabrous hindpaw skin (red chevrons). I–L) Sham-operated, non-CCI rats have epidermal keratinocyte expression of CGRP that is heterogeneous and weak among the volar pad crown (I, red chevrons), with intense CGRP immunolabeling of a subset of PGP-positive axonal innervation (I, red arrows; J, PGP positive, red arrows) among the epidermal endings (downward red arrows), and upper dermal fibers (upward red arrows), and within dermal nerves (K, red panel). NF content within the dermal nerves was extensively distributed among mostly the larger caliber axons and some small caliber axons (L, red panel). M–P) CCI rats showed dramatically elevated expression of CGRP among the suprabasal keratinocytes (M, red chevrons), coincident with depleted CGRP-IL epidermal endings and dermal nerve fibers, among an overall depleted innervation (N, PGP positive, red arrows). Interestingly, CGRP content appears to be increased within deep dermal nerves (O, red panel), but was mostly distributed among vacuolated cells affiliated with the larger caliber axons. NF-IL is severely depleted among the large caliber axons within the deep nerves, indicating that these axons may be deteriorating and that the vacuolated cells may be reactive Schwann cells (P, red panel). Magnification bar (D) =50µm for C–H, and I,J,M,N; (K)=25µm for K,L,O,P.
Figure 3. Hindpaw inflammation increases CGRP expression in epidermal keratinocytes
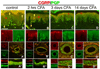
A–D) CFA hindpaw injection time course, depicting in each vertical panel, representative double label (CGRP/PGP) results from glabrous pad crown (A–D), dermal vasculature (‘), and dermal nerve (‘’), with each separate channel split below or to the left of the double label image. A) Control uninjected rats depicting CGRP-IL of a subset of innervation (PGP-IL, green) among the epidermal endings and subepidermal plexus (yellow arrows). The separate channels are split below the double labeled images. Epidermal keratinocyte CGRP-IL (red chevrons) appeared heterogeneous and weak among the volar pad crown. A’) Neural innervation to dermal vasculature contained a mix of CGRP-IL-positive and CGRP-IL-negative axons among the tunica media and advential layers. A”) Dermal nerves contained a rich contingent of CGRP-IL axons. B) By 2 hrs after CFA injection, increased CGRP expression is apparent among epidermal keratinocytes, with little change among the innervation of the epidermis, subepidermal plexus, dermal vasculature (B’), or in the composition of dermal nerves (B”). C) By 3 days after CFA injection, epidermal CGRP expression is increased among suprabasal keratinocytes, while neural innervation appears depleted (i.e., lack of CGRP or PGP axon staining) from the epidermis and subepidermal plexus. C’) Interestingly, among the dermal vasculature, a dramatic loss of CGRP immunopositive medial and adventitial innervation is observed, although PGP immunopositive axons are still present. Dermal nerves appear distraught with ragged PGP labeling and limited CGRP axons present (C”). D) By 14 days following CFA, epidermal CGRP expression remains elevated among the most suprabasal keratinocytes, and immunopositive axons are absent from the epidermis or subepidermal plexus. D’) Dermal vasculature appeared without CGRP immunopositive innervation among the medial and adventitial layers, although PGP immunopositive axons were still present, and dermal nerves remained distraught in appearance with limited CGRP axons (D”). Magnification bar (A) =50µm for A–D, and =25µm for (‘) and (‘’). Anti-CGRP (guinea pig polyclonal, 1:800; Peninsula Labs, San Carlos, CA) and anti-PGP (rabbit polyclonal, 1:1000; UltraClone, Wellow, UK) were used.
Figure 4. SIV infection in rhesus macaques results in an increase of CGRP-IL in epidermal keratinocytes
A–E) CGRP-IL is increased among palmar glabrous skin keratinocytes from SIV-infected animals compared with control epidermis. A) Control monkey epidermal primary ridge depicting CGRP-IL among fine delicate innervation of the epidermis, and upper dermal compartments (downward and upward arrows, respectively). B–E) Compared with the control monkey, all monkeys infected with SIV had increased CGRP-IR, although the intensity varied between monkeys. B) As seen in an aged monkey with AIDS and severe SIV encephalitis (SIVE), moderate CGRP-IL is detected among the epidermal keratinocytes. C) This young adult monkey had no AIDS defining illness, but did show abundant systemic viral replication and had severe colitis at the time of sacrifice. Dramatic increases in CGRP-IL of keratinocytes can be seen. D) This represents a similarly young adult monkey with SIV infection, with the most subtle increase in epidermal keratinocyte CGRP-IL observed. In this animal, CGRP-IL among keratinocytes was only slightly higher compared with the control or other SIV animals, and CGRP-IL innervation was observed (arrows). E) This represents another young adult SIV infected animal, demonstrating robust CGRP-IL of epidermal keratinocytes, and CGRP-IL among upper dermal nerves (arrow). Magnification bar (A) =50µm for A–E. Anti-CGRP (guinea pig polyclonal, 1:800; Peninsula Labs, San Carlos, CA) was used.
Figure 5. Epidermal keratinocyte CGRP-IL is effected by the noggin/BMP4 system
Utilizing transgenic mice which over-produce noggin or BMP4 driven off of the K14 promoter, hindpaw skin was removed and immunolabeled for CGRP. A,B) Noggin over-expression results in intense CGRP-IL in keratinocytes among the volar pad crown (A) and flat (B) glabrous hindpaw skin, appearing more extensive throughout suprabasal keratinocytes. C,D) BMP4 over-expression results in little CGRP-IL in keratinocytes among the volar pad crown (E) and flat (F) glabrous hindpaw skin. The BMP4 keratinocyte expression appeared heterogeneous and similar to that observed in control rats (see Fig. 2C, I). Note that CGRP immunolabeling on nerve fibers was present in the skin of both noggin and BMP4 over-expressing mice, although a prior study detected differences among subtypes of CGRP-positive fibers. Magnification bar =50µm. Anti-CGRP (rabbit polyclonal, 1:1000; Chemicon Inc, Temecula, CA) was used.
Figure 6. CGRP mRNA expression in transgenic mouse epidermal keratinocytes in vivo, and normal human keratinocytes in vitro
Real time quantitative PCR was performed using primers specific to CGRPα, CGRPβ, BMP4, noggin, and members of the CGRP receptor complex, CRLR, RAMP1, and RCP. A–C) Data is presented as relative mRNA levels, normalized to the reference gene GAPDH. A) Epidermal glabrous skin compartments were obtained by laser capture microdissection from BMP4 or noggin over-expression transgenic mice. Total mRNA was obtained from a pool of 5–7 etched epidermis’ per sample. Transgenic over-expression of noggin results in significantly greater expression of CGRPα and CGRPβ mRNA compared with the BMP4 over-expressing transgenic mice (*). As well, the CGRPβ isoform is expressed in significantly greater amounts (~3.5-fold) than the CGRPα isoform among both lines of transgenic mice (#). B) Normal human epidermal keratinocytes (NHEK) were cultured with standard media (undiff), or calcium differentiated with 2M CaCl2 (diff), and primary human keratinocytes were grown as a 3D raft culture at the air interface, allowing for stratification and formation of an oragnotypic psudoepidermis (3D raft). Keratinocyte differentiation significantly increases the production of both CGRP mRNA isoforms compared with undifferentiated NHEK cells (*). Importantly, under all three keratinocyte growth conditions, data demonstrates that the CGRPβ isoform is expressed in undifferentiated NHEK (10.4-fold increase), differentiated NHEK (8.0-fold increase), and 3D raft cultures (12.3 fold increase), to a significantly greater degree than the CGRPα isoform (#). C) Under all keratinocyte growth conditions, the complete receptor complex components specific for CGRP were detected. For A–C, * and # p<0.05 by Student’s t-test.
Figure 7. CGRP precursor protein is expressed in mouse and human keratinocytes using western blot
Total protein was isolated from primary and immortalized mouse keratinocytes (MK116), three-dimensional cultured human epidermal keratinocytes (3D raft cultures), cultured mouse fibroblasts (FB), and mouse trigeminal ganglia (TG). Western blot analysis was performed to determine CGRP protein content. A) Duplicate protein samples from primary mouse keratinocytes were loaded at 20, 40, and 60 µg/lane, along with CGRPα positive control lanes, separated, transferred to PDVF nylon membranes and probed with anti-CGRP (Rb polyclonal, 1:2000; Chemicon Inc, Temecula, CA). A low level detectable band was observed from all samples at the predicted weight of CGRP precursor protein (16kDa; arrows). The positive control CGRPα was detected at the processed size (~3.7kD), however, among the in vitro cultures, no mature peptide was detectable. B) Total protein samples from mouse fibroblasts (FB, as negative control), immortalized mouse keratinocytes (MK116), and mouse trigeminal ganglia (TG, as positive control) were loaded (20 µg/lane), separated, transferred to PDVF nylon membranes and probed with anti-CGRP (Rb polyclonal, 1:2000; Chemicon Inc, Temecula, CA). A detectable band corresponding to the predicted molecular weight of the CGRP precursor protein (16kD; arrows) was observed in the keratinocyte and trigeminal ganglia lanes, but was not detectable in the fibroblast lane. C) Total protein samples from organotypic human keratinocytes (3D raft cultures) were loaded, along with CGRPα positive control, separated, transferred to PDVF nylon membranes and probed with anti-CGRP (Rb polyclonal, 1:2000; Chemicon Inc, Temecula, CA). A detectable band corresponding to the predicted molecular weight of the CGRP precursor protein (16kD; arrows) was observed in all lanes. D) Total protein samples from mouse trigeminal ganglia (TG, positive control) and mouse fibroblasts (FB, negative control) were loaded (20 µg/lane), along with CGRPα (1µg) positive control, in duplicate, separated, transferred to PDVF nylon membranes, and the membrane then separated. One half of the membrane (left) was then probed with anti-CGRP (Rb polyclonal, 1:2000; Chemicon Inc, Temecula, CA), while the other half (right) was probed with pre-adsorbed anti-CGRP antibody. Mature CGRP (~3.7kD) and CGRP precursor protein (16kD; arrows) bands were detected on the left membrane, and no bands were detectible on the right membrane after specific primary antibody pre-adsorption.
Similar articles
- Keratinocytes express cytokines and nerve growth factor in response to neuropeptide activation of the ERK1/2 and JNK MAPK transcription pathways.
Shi X, Wang L, Clark JD, Kingery WS. Shi X, et al. Regul Pept. 2013 Sep 10;186:92-103. doi: 10.1016/j.regpep.2013.08.001. Epub 2013 Aug 17. Regul Pept. 2013. PMID: 23958840 Free PMC article. - Epidermal nerve fibers modulate keratinocyte growth via neuropeptide signaling in an innervated skin model.
Roggenkamp D, Köpnick S, Stäb F, Wenck H, Schmelz M, Neufang G. Roggenkamp D, et al. J Invest Dermatol. 2013 Jun;133(6):1620-8. doi: 10.1038/jid.2012.464. Epub 2013 Jan 3. J Invest Dermatol. 2013. PMID: 23283070 - Post-junctional facilitation of Substance P signaling in a tibia fracture rat model of complex regional pain syndrome type I.
Wei T, Li WW, Guo TZ, Zhao R, Wang L, Clark DJ, Oaklander AL, Schmelz M, Kingery WS. Wei T, et al. Pain. 2009 Aug;144(3):278-286. doi: 10.1016/j.pain.2009.04.020. Epub 2009 May 21. Pain. 2009. PMID: 19464118 Free PMC article. - The Effects of Calcitonin Gene-Related Peptide on Bone Homeostasis and Regeneration.
Xu J, Wang J, Chen X, Li Y, Mi J, Qin L. Xu J, et al. Curr Osteoporos Rep. 2020 Dec;18(6):621-632. doi: 10.1007/s11914-020-00624-0. Epub 2020 Oct 8. Curr Osteoporos Rep. 2020. PMID: 33030684 Review. - [Regulatory mechanisms of calcitonin gene-related peptide (CGRP) in skin inflammation].
Mikami N, Fukada S, Yamamoto H, Tsujikawa K. Mikami N, et al. Yakugaku Zasshi. 2012;132(11):1211-5. doi: 10.1248/yakushi.12-00232-1. Yakugaku Zasshi. 2012. PMID: 23123709 Review. Japanese.
Cited by
- Modulatory role of sensory innervation on hair follicle stem cell progeny during wound healing of the rat skin.
Martínez-Martínez E, Galván-Hernández CI, Toscano-Márquez B, Gutiérrez-Ospina G. Martínez-Martínez E, et al. PLoS One. 2012;7(5):e36421. doi: 10.1371/journal.pone.0036421. Epub 2012 May 4. PLoS One. 2012. PMID: 22574159 Free PMC article. - Keratinocyte expression of inflammatory mediators plays a crucial role in substance P-induced acute and chronic pain.
Wei T, Guo TZ, Li WW, Hou S, Kingery WS, Clark JD. Wei T, et al. J Neuroinflammation. 2012 Jul 23;9:181. doi: 10.1186/1742-2094-9-181. J Neuroinflammation. 2012. PMID: 22824437 Free PMC article. - Exploring Novel Therapeutic Targets in the Common Pathogenic Factors in Migraine and Neuropathic Pain.
Tajti J, Szok D, Csáti A, Szabó Á, Tanaka M, Vécsei L. Tajti J, et al. Int J Mol Sci. 2023 Feb 18;24(4):4114. doi: 10.3390/ijms24044114. Int J Mol Sci. 2023. PMID: 36835524 Free PMC article. Review. - Endothelin receptor type A is involved in the development of oxaliplatin-induced mechanical allodynia and cold allodynia acting through spinal and peripheral mechanisms in rats.
Matsuura K, Sakai A, Watanabe Y, Mikahara Y, Sakamoto A, Suzuki H. Matsuura K, et al. Mol Pain. 2021 Jan-Dec;17:17448069211058004. doi: 10.1177/17448069211058004. Mol Pain. 2021. PMID: 34894846 Free PMC article. - Therapeutic effect of human adipose-derived stem cells and their secretome in experimental diabetic pain.
Brini AT, Amodeo G, Ferreira LM, Milani A, Niada S, Moschetti G, Franchi S, Borsani E, Rodella LF, Panerai AE, Sacerdote P. Brini AT, et al. Sci Rep. 2017 Aug 29;7(1):9904. doi: 10.1038/s41598-017-09487-5. Sci Rep. 2017. PMID: 28851944 Free PMC article.
References
- Ai X, Cappuzzello J, Hall AK. Activin and bone morphogenetic proteins induce calcitonin gene-related peptide in embryonic sensory neurons in vitro. Mol Cell Neurosci. 1999;14:506–518. - PubMed
- Albrecht PJ, Hines S, Eisenberg E, Pud D, Finlay DR, Connolly MK, Pare M, Davar G, Rice FL. Pathologic alterations of cutaneous innervation and vasculature in affected limbs from patients with complex regional pain syndrome. Pain. 2006;120:244–266. - PubMed
- Albrecht PJ, Rice FL. Role of small-fiber afferents in pain mechanisms with implications on diagnosis and treatment. Curr Pain Headache Rep. 2010;14:179–188. - PubMed
- Ambalavanar R, Dessem D, Moutanni A, Yallampalli C, Yallampalli U, Gangula P, Bai G. Muscle inflammation induces a rapid increase in calcitonin gene-related peptide (CGRP) mRNA that temporally relates to CGRP immunoreactivity and nociceptive behavior. Neuroscience. 2006;143:875–884. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- P30 AR057216/AR/NIAMS NIH HHS/United States
- 1P30AR057216-01/AR/NIAMS NIH HHS/United States
- T32 DA007307/DA/NIDA NIH HHS/United States
- R01 NS020778-26/NS/NINDS NIH HHS/United States
- R01 NS020778/NS/NINDS NIH HHS/United States
- T32 DA007307-03/DA/NIDA NIH HHS/United States
- DA07307/DA/NIDA NIH HHS/United States
- R01 NS020013/NS/NINDS NIH HHS/United States
- R01 NS020013-26/NS/NINDS NIH HHS/United States
- R01 NS020013-27/NS/NINDS NIH HHS/United States
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials