Laminar fate specification in the cerebral cortex - PubMed (original) (raw)
Laminar fate specification in the cerebral cortex
Nicolas Gaspard et al. F1000 Biol Rep. 2011.
Abstract
The cerebral cortex is composed of hundreds of different types of neurons, which underlie its ability to perform highly complex neural processes. How this astonishing cell diversity is generated during development constitutes a major challenge in developmental neurosciences, with important implications for neurological diseases. Here we review some recent and exciting advances in this field, from the description of the cellular processes at the origin of cortical neuron diversity, to the dissection of the molecular logic underlying fate selection in cortical neurons.
Figures
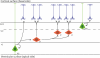
Figure 1.. Laminar organization and pattern of cortical projections
Simplified scheme depicting the laminar organization of the cortex in terms of gene expression and axonal projections. Neurons sending corticofugal projections (in blue) reside exclusively in the deep layers 5 and 6 of the cortex, while those sending projections within the cortex (in red), including callosal projections to the contralateral side, reside primarily in the upper layers, with a small contingent of callosal-projecting neurons in layer 5. Each subtype expresses specific combinations of transcription factors (in blue and red).
Figure 2.. Diversity of cortical progenitors
Several types of cortical progenitors and their modes of division towards neurons (in blue) are depicted, including radial glial cells (RG), intermediate progenitors (IP), and outer radial glial cells (ORG), with their specific location in ventricular (VZ), subventricular (SVZ) or outer subventricular (OSVZ) zones.
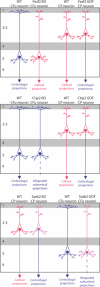
Figure 3.. From genes to neuronal fates
Scheme depicting the axonal projections of cortical neurons in normal (wild-type) conditions (WT) or following knockout (KO) or gain-of-function (GOF) of three major transcription factors involved in fate specification: Fezf2, Ctip2, and Satb2. See text for further explanation.
Similar articles
- Cortical neurogenesis and morphogens: diversity of cues, sources and functions.
Tiberi L, Vanderhaeghen P, van den Ameele J. Tiberi L, et al. Curr Opin Cell Biol. 2012 Apr;24(2):269-76. doi: 10.1016/j.ceb.2012.01.010. Epub 2012 Feb 17. Curr Opin Cell Biol. 2012. PMID: 22342580 Review. - Molecular mechanisms of projection neuron production and maturation in the developing cerebral cortex.
Mérot Y, Rétaux S, Heng JI. Mérot Y, et al. Semin Cell Dev Biol. 2009 Aug;20(6):726-34. doi: 10.1016/j.semcdb.2009.04.003. Epub 2009 Apr 10. Semin Cell Dev Biol. 2009. PMID: 19442543 Review. - Development and regeneration of projection neuron subtypes of the cerebral cortex.
Tomassy GS, Lodato S, Trayes-Gibson Z, Arlotta P. Tomassy GS, et al. Sci Prog. 2010;93(Pt 2):151-69. doi: 10.3184/003685010X12705764469952. Sci Prog. 2010. PMID: 20681320 Free PMC article. Review. - Wnt/beta-catenin signaling in cerebral cortical development.
Chenn A. Chenn A. Organogenesis. 2008 Apr;4(2):76-80. doi: 10.4161/org.4.2.5852. Organogenesis. 2008. PMID: 19279718 Free PMC article. - Fate bias during neural regeneration adjusts dynamically without recapitulating developmental fate progression.
Ng Chi Kei J, Currie PD, Jusuf PR. Ng Chi Kei J, et al. Neural Dev. 2017 Jul 13;12(1):12. doi: 10.1186/s13064-017-0089-y. Neural Dev. 2017. PMID: 28705258 Free PMC article.
Cited by
- Human induced pluripotent stem cell/embryonic stem cell-derived pyramidal neuronal precursors show safety and efficacy in a rat spinal cord injury model.
Li M, Qi B, Li Q, Zheng T, Wang Y, Liu B, Guan Y, Bai Y, Jian F, Xu ZD, Xu Q, Chen Z. Li M, et al. Cell Mol Life Sci. 2024 Jul 29;81(1):318. doi: 10.1007/s00018-024-05350-9. Cell Mol Life Sci. 2024. PMID: 39073571 Free PMC article. - MKL/SRF and Bcl6 mutual transcriptional repression safeguards the fate and positioning of neocortical progenitor cells mediated by RhoA.
Cossard A, Stam K, Smets A, Jossin Y. Cossard A, et al. Sci Adv. 2023 Nov 17;9(46):eadd0676. doi: 10.1126/sciadv.add0676. Epub 2023 Nov 15. Sci Adv. 2023. PMID: 37967194 Free PMC article. - Increased Nuclear FOXP2 Is Related to Reduced Neural Stem Cell Number and Increased Neurogenesis in the Dorsal Telencephalon of Embryos of Diabetic Rats through Histamine H1 Receptors.
De la Merced-García DS, Sánchez-Barrera Á, Hernández-Yonca J, Mancilla I, García-López G, Díaz NF, Terrazas LI, Molina-Hernández A. De la Merced-García DS, et al. Cells. 2023 Feb 3;12(3):510. doi: 10.3390/cells12030510. Cells. 2023. PMID: 36766852 Free PMC article. - Creative Destruction: A Basic Computational Model of Cortical Layer Formation.
Bauer R, Clowry GJ, Kaiser M. Bauer R, et al. Cereb Cortex. 2021 Jun 10;31(7):3237-3253. doi: 10.1093/cercor/bhab003. Cereb Cortex. 2021. PMID: 33625496 Free PMC article. - An architectonic type principle in the development of laminar patterns of cortico-cortical connections.
Beul SF, Goulas A, Hilgetag CC. Beul SF, et al. Brain Struct Funct. 2021 May;226(4):979-987. doi: 10.1007/s00429-021-02219-6. Epub 2021 Feb 9. Brain Struct Funct. 2021. PMID: 33559742 Free PMC article.
References
- Noctor SC, Martinez-Cerdeno V, Ivic L, Kriegstein AR. Cortical neurons arise in symmetric and asymmetric division zones and migrate through specific phases. Nat Neurosci. 2004;7:136–44. doi: 10.1038/nn1172. - DOI - PubMed
- F1000 Factor 11
Evaluated by Gordon Fishell 23 Feb 2004, Jaime García-Añoveros 16 Apr 2004, Francois Guillemot 02 Jul 2004
LinkOut - more resources
Full Text Sources