Latent TGF-β structure and activation - PubMed (original) (raw)
Latent TGF-β structure and activation
Minlong Shi et al. Nature. 2011.
Abstract
Transforming growth factor (TGF)-β is stored in the extracellular matrix as a latent complex with its prodomain. Activation of TGF-β1 requires the binding of α(v) integrin to an RGD sequence in the prodomain and exertion of force on this domain, which is held in the extracellular matrix by latent TGF-β binding proteins. Crystals of dimeric porcine proTGF-β1 reveal a ring-shaped complex, a novel fold for the prodomain, and show how the prodomain shields the growth factor from recognition by receptors and alters its conformation. Complex formation between α(v)β(6) integrin and the prodomain is insufficient for TGF-β1 release. Force-dependent activation requires unfastening of a 'straitjacket' that encircles each growth-factor monomer at a position that can be locked by a disulphide bond. Sequences of all 33 TGF-β family members indicate a similar prodomain fold. The structure provides insights into the regulation of a family of growth and differentiation factors of fundamental importance in morphogenesis and homeostasis.
Figures
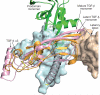
Figure 1. Architecture of proTGF-β1
Arm, straitjacket and TGF-β1 monomer segments are coloured differently.a, b, Overall structure. Spheres mark the last residue visible in density in the prodomain and the first residue of the growth factor. Disordered segments are dashed. Red arrows show the directions of forces during activation by integrins. Key side chains are shown in stick representation, including Asp of the RGD motif in cyan and CED mutations in white. Disulphide bonds and the Cys 4 mutation to Ser are in yellow.c, Schematic of the structure and activation mechanism. SS, disulphide bonds. d, Hydrophobic residues near Asp 217 of the RGD motif. e, Arm domain. Side chains for the hydrophobic core are shown in gold (also marked in Fig. 2 and Supplementary Fig. 2), conserved α2-helix residues that interact with the growth factor are in pink, fastener residues are in silver and bowtie residues are in light green for aliphatics and yellow for Cys. f–h, Straitjacket and fastener details.
Figure 2. The TGF-β family
a, Sequence alignment of five representative prodomains. Orange circles mark the core hydrophobic residues shown in Fig. 1e. Inh-α, inhibin-α; Myost, myostatin. Black dots over TGF-β1 sequence mark decadal residues. Vertical dashed lines mark cleavage sites. b, Phylogenetic tree of the TGF-β family, based on the alignment in Supplementary Fig. 2.
Figure 3. Shielding from receptor binding
ProTGF-β1 and TGF-β1 in complex with its receptors (R type I and II) (ref. 15) were superimposed on the TGF-β dimers. For clarity, only one monomer of each is shown. The receptors are shown as transparent molecular surfaces. Elements of the prodomain that clash with the receptors are labelled.
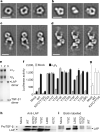
Figure 4. ProTGF-β1 complexes with LTBP and and αvβ6 integrin, and activation of TGF-β
a–d, Representative negative-stain electron microscopy class averages of proTGF-β (a), the complex of proTGF-β with a fragment of LTBP1 containing TGF-βinding domain 3–EGF–EGF–TGF-βinding domain 4 (b) and complexes of proTGF-β1 with αvβ6integrin, prepared with an excess of proTGF-β1 (c) or an excess of αvβ6 integrin (d). Scale bars, 100Å. e, Non-reducing SDS–PAGE of the complex peak from S200 gel filtration used for electron microscopy in d (lane 1), αvβ6 integrin (lane 2) and proTGF-β (lane 3). LAP, latency-associated protein (prodomain).f, Activation of TGF-β1. 293T cells stably transfected with αvβ6 integrin or a mock control were additionally transfected with the indicated wild-type (WT) or mutant proTGF-β1 constructs, or with empty vector (mock), and co-cultured with TGF-β indicator cells.g, Material made by the indicated mutants in 293T cells was heated at 80 °C and assayed with indicator cells. Error bars inf and g show the s.e.m. of 3–9 samples from 1–3 representative experiments. h, i, Western blots of proTGF-β1 secreted by the indicated transfectants, using an antibody to the prodomain (h) or streptavidin to detect biotinylated cysteines (i).
Similar articles
- Force interacts with macromolecular structure in activation of TGF-β.
Dong X, Zhao B, Iacob RE, Zhu J, Koksal AC, Lu C, Engen JR, Springer TA. Dong X, et al. Nature. 2017 Feb 2;542(7639):55-59. doi: 10.1038/nature21035. Epub 2017 Jan 25. Nature. 2017. PMID: 28117447 Free PMC article. - Prodomain-growth factor swapping in the structure of pro-TGF-β1.
Zhao B, Xu S, Dong X, Lu C, Springer TA. Zhao B, et al. J Biol Chem. 2018 Feb 2;293(5):1579-1589. doi: 10.1074/jbc.M117.809657. Epub 2017 Nov 5. J Biol Chem. 2018. PMID: 29109152 Free PMC article. - Structural determinants of integrin β-subunit specificity for latent TGF-β.
Dong X, Hudson NE, Lu C, Springer TA. Dong X, et al. Nat Struct Mol Biol. 2014 Dec;21(12):1091-6. doi: 10.1038/nsmb.2905. Epub 2014 Nov 10. Nat Struct Mol Biol. 2014. PMID: 25383667 Free PMC article. - Integrins and the activation of latent transforming growth factor beta1 - an intimate relationship.
Wipff PJ, Hinz B. Wipff PJ, et al. Eur J Cell Biol. 2008 Sep;87(8-9):601-15. doi: 10.1016/j.ejcb.2008.01.012. Epub 2008 Mar 14. Eur J Cell Biol. 2008. PMID: 18342983 Review. - Latent transforming growth factor-beta binding proteins (LTBPs)--structural extracellular matrix proteins for targeting TGF-beta action.
Saharinen J, Hyytiäinen M, Taipale J, Keski-Oja J. Saharinen J, et al. Cytokine Growth Factor Rev. 1999 Jun;10(2):99-117. doi: 10.1016/s1359-6101(99)00010-6. Cytokine Growth Factor Rev. 1999. PMID: 10743502 Review.
Cited by
- Bifunctional TGF-β trap/IL-15 protein complex elicits potent NK cell and CD8+ T cell immunity against solid tumors.
Liu B, Zhu X, Kong L, Wang M, Spanoudis C, Chaturvedi P, George V, Jiao JA, You L, Egan JO, Echeverri C, Gallo VL, Xing J, Ravelo K, Prendes C, Antolinez J, Denissova J, Muniz GJ, Jeng EK, Rhode PR, Wong HC. Liu B, et al. Mol Ther. 2021 Oct 6;29(10):2949-2962. doi: 10.1016/j.ymthe.2021.06.001. Epub 2021 Jun 4. Mol Ther. 2021. PMID: 34091051 Free PMC article. - Reducing affinity of αvβ8 interactions with latent TGFβ: dialling down fibrosis.
Tatler AL, Jenkins G. Tatler AL, et al. Ann Transl Med. 2015 May;3(Suppl 1):S31. doi: 10.3978/j.issn.2305-5839.2015.02.18. Ann Transl Med. 2015. PMID: 26046078 Free PMC article. No abstract available. - Latent TGF-β-binding proteins.
Robertson IB, Horiguchi M, Zilberberg L, Dabovic B, Hadjiolova K, Rifkin DB. Robertson IB, et al. Matrix Biol. 2015 Sep;47:44-53. doi: 10.1016/j.matbio.2015.05.005. Epub 2015 May 8. Matrix Biol. 2015. PMID: 25960419 Free PMC article. Review. - On-Target Anti-TGF-β Therapies Are Not Succeeding in Clinical Cancer Treatments: What Are Remaining Challenges?
Teixeira AF, Ten Dijke P, Zhu HJ. Teixeira AF, et al. Front Cell Dev Biol. 2020 Jul 8;8:605. doi: 10.3389/fcell.2020.00605. eCollection 2020. Front Cell Dev Biol. 2020. PMID: 32733895 Free PMC article. Review. - A 3D Poly(ethylene glycol)-based Tumor Angiogenesis Model to Study the Influence of Vascular Cells on Lung Tumor Cell Behavior.
Roudsari LC, Jeffs SE, Witt AS, Gill BJ, West JL. Roudsari LC, et al. Sci Rep. 2016 Sep 6;6:32726. doi: 10.1038/srep32726. Sci Rep. 2016. PMID: 27596933 Free PMC article.
References
- Wu MY, Hill CS. TGF-β superfamily signaling in embryonic development and homeostasis. Dev. Cell. 2009;16:329–343. - PubMed
- Derynck R, Miyazono K. In: The TGF-β Family. Derynck R, Miyazono K, editors. Cold Spring Harbor Laboratory Press; 2008. pp. 29–43. Ch. 2.
- Blobe GC, Schiemann WP, Lodish HF. Role of transforming growth factor β in human disease. N. Engl. J. Med. 2000;342:1350–1358. - PubMed
- Gray AM, Mason AJ. Requirement for activin A and transforming growth factor-β 1 pro-regions in homodimer assembly. Science. 1990;247:1328–1330. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases