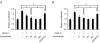Sunitinib inhibits lymphatic endothelial cell functions and lymph node metastasis in a breast cancer model through inhibition of vascular endothelial growth factor receptor 3 - PubMed (original) (raw)
Sunitinib inhibits lymphatic endothelial cell functions and lymph node metastasis in a breast cancer model through inhibition of vascular endothelial growth factor receptor 3
Yasuo Kodera et al. Breast Cancer Res. 2011.
Abstract
Introduction: Metastasis is a common event and the main cause of death in cancer patients. Lymphangiogenesis refers to the formation of new lymphatic vessels and is thought to be involved in the development of metastasis. Sunitinib is a multi-kinase inhibitor that blocks receptor tyrosine kinase activity, including that of vascular endothelial growth factor receptors (VEGFRs). Although sunitinib is a clinically available angiogenesis inhibitor, its effects on lymphangiogenesis and lymph node metastasis remain unclear. The purpose of this study was to investigate the effects of sunitinib on vascular endothelial growth factor receptor 3 (VEGFR-3) and a related event, lymphangiogenesis.
Methods: The effects of sunitinib on the degree of phosphorylation of VEGFR-2/3 and other signaling molecules was examined in lymphatic endothelial cells (LECs) treated with the drug; VEGF-induced LEC growth, migration, and tube formation were also examined. For the in vivo study, luciferase-expressing breast cancer cells were transplanted into mammary fat pads of mice; the microvessel and lymphatic vessel density was then measured after treatment with sunitinib and anti-VEGFR-2 antibody.
Results: First, in human LECs, sunitinib blocked both VEGFR-2 and VEGFR-3 phosphorylation induced by VEGF-C or VEGF-D, and abrogated the activation of the downstream molecules extracellular signal-regulated kinase 1/2 (ERK1/2) and Akt. Furthermore, sunitinib attenuated the cell-proliferation activity induced by VEGF-C/D and prevented VEGF-C-induced migration and tube formation of the LECs; however, anti-VEGFR2 treatment shows only a partial effect on the growth and functions of the LECs. We used a breast cancer cell line expressing luciferase as a metastatic cancer model. Sunitinib treatment (40 mg/kg/day) inhibited the growth of the primary tumor transplanted in the mammary fat pad of the mice and significantly reduced the number of blood and lymphatic vessels in the tumor. Furthermore, the development of axillary lymph node metastasis, detected by bioluminescent imaging, was markedly suppressed. This effect of sunitinib was more potent than that of DC101, an anti-mouse VEGFR-2 antibody.
Conclusions: The results suggest that sunitinib might be beneficial for the treatment of breast cancer by suppressing lymphangiogenesis and lymph node metastasis, through inhibition, particularly important, of VEGFR-3.
Figures
Figure 1
Sunitinib blocked activation of VEGFR-2 and VEGFR-3 and their downstream molecules in human dermal LECs. LECs were cultured to confluence, and the medium was replaced with a serum-free medium. After overnight incubation, the cells were pretreated with sunitinib, anti-VEGFR2 antibody, or DMSO (controls), before being stimulated with 200 ng/ml VEGF-C (a) or VEGF-D (b). Lysates were immunoprecipitated with VEGFR-3-specific antibody for phospho-VEGFR-3 detection (c, d). Other phosphoproteins were detected with their respective specific antibodies. All samples were separated by 4% to 20% gradient SDS-PAGE gel. The proteins were blotted onto a PVDF membrane and detected by Western blotting. Tyrosine phosphorylation of VEGFR-2 and VEGFR-3 was noted in the cells treated with VEGF-C or VEGF-D. Sunitinib blocked phosphorylation of both VEGFR-3 and VEGFR-2, whereas anti-VEGFR-2 antibody inhibited phosphorylation of only VEGFR-2. Increased phosphorylation of ERK1/2 and Akt was observed in the LECs stimulated with the VEGFs; however, this was attenuated in the cells treated with sunitinib. DMSO, dimethyl sulfoxide; LEC, lymphatic endothelial cell; MW, molecular weight marker; VEGF, vascular endothelial growth factor; VEGFR, vascular endothelial growth factor receptor.
Figure 2
Sunitinib inhibited VEGF-induced proliferation of LECs. LECs were incubated in serum-free conditions in the absence or presence of 500 ng/ml of VEGF-C (left) or VEGF-D (right), and DMSO or 10 to 300 n_M_ sunitinib. The cellular proliferation was quantified by MTS assay after 7 days of treatment. Columns indicate the values in six replicates normalized to the nontreated controls; bars, SD. *P < 0.05 compared with control. DMSO, dimethyl sulfoxide; LEC, lymphatic endothelial cell; VEGF, vascular endothelial growth factor; VEGFR, vascular endothelial growth factor receptor.
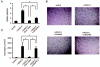
Figure 3
Sunitinib inhibited VEGF-C-induced functions of LECs. (a) Quantification of LEC migration induced by VEGF-C in cells treated with DMSO or 10 nmol/ml of sunitinib. Relative migration indicates the number of migrated cells normalized to the number of control cells. Columns indicate the values from four experiments; bars, SD. *P < 0.05 as compared with control. Sunitinib suppressed VEGF-C-induced LEC migration. (b) Photographs of VEGF-C-induced tube formation in cells treated with sunitinib or DMSO (control). VEGF-C promoted cord formation after overlay of type I collagen, which was suppressed by sunitinib treatment, but not by anti-VEGFR2 antibody. The black line represents 200 μm. (c) Quantification of tube formation. Columns indicate the values from the data of four fields; bars, SD. Sunitinib inhibited VEGF-C-induced tube formation. *P < 0.05 as compared with control. DMSO, dimethyl sulfoxide; LEC, lymphatic endothelial cell; Suni, sunitinib; VEGF, vascular endothelial growth factor; VEGFR, vascular endothelial growth factor receptor.
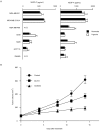
Figure 4
Expression of VEGF-C in various cancer cell lines; sunitinib inhibited MDA-MB-231LN tumor growth. (a) VEGF expression levels were quantified in metastatic cancer cell lines under normoxic and hypoxic conditions. All cells were cultured overnight under normoxic or hypoxic (5% O2) conditions; subsequently, the amounts of VEGFs secreted in the conditioned medium were quantified with enzyme-linked immunosorbent assay (a). Columns indicate the values of four replicates; bars, SD. (b) Effects of sunitinib and of the anti-VEGFR-2 antibody, DC101, on the primary tumor growth. MDA-MB-231LN cells were transplanted into the m.f.p. of female mice, and the treatment was initiated on day 5 after the inoculation. Sunitinib was administered orally at 40 mg/kg once a day, and DC101 at 800 μg/mouse i.p. every 3 days for 2 weeks. Tumor volume was measured on the indicated days. Points indicate tumor volume; bars, SEM. *P < 0.05 as compared with control. m.f.p., mammary fat pad; N.D., not detected; VEGF, vascular endothelial growth factor; VEGFR, vascular endothelial growth factor receptor.
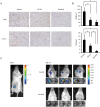
Figure 5
Sunitinib inhibited angiogenesis, lymphangiogenesis, and lymph node metastasis in the m.f.p. model. (a) Immunohistochemical analysis of the primary tumor treated with sunitinib, DC101, or vehicle. Tumors were stained with anti-CD31 for endothelial cells (upper) and anti-LYVE-1 for lymphatic endothelial cells (lower). Photographs were taken under a microscope at a magnification of ×8. Photographs of representative areas are shown. The black line represents 50 μm. (b) Effects of sunitinib and DC101 on tumor angiogenesis and lymphangiogenesis. The microvessel density (MVD, top) was determined by staining for CD31, and the lymphatic microvessel density (LVD, bottom) by staining for LYVE-1. *P < 0.05 as compared with control. (c) Lymph node metastasis was detected by bioluminescent imaging in vivo. The primary tumor (left) was identified on day 1 of treatment, and tumor metastasis around the axillary lymph node was detected after treatment with vehicle control (right, upper) or sunitinib (right, lower). Ex vivo data of the lymph nodes confirmed metastasis from the MDA-MB-231LN primary tumor. Three representative photographs from each treatment group are shown. LVD, lymphatic microvessel density; m.f.p., mammary fat pad; MVD, microvessel density.
Similar articles
- The tyrosine kinase inhibitor cediranib blocks ligand-induced vascular endothelial growth factor receptor-3 activity and lymphangiogenesis.
Heckman CA, Holopainen T, Wirzenius M, Keskitalo S, Jeltsch M, Ylä-Herttuala S, Wedge SR, Jürgensmeier JM, Alitalo K. Heckman CA, et al. Cancer Res. 2008 Jun 15;68(12):4754-62. doi: 10.1158/0008-5472.CAN-07-5809. Cancer Res. 2008. PMID: 18559522 - Targeting VEGFR-3/-2 signaling pathways with AD0157: a potential strategy against tumor-associated lymphangiogenesis and lymphatic metastases.
García-Caballero M, Paupert J, Blacher S, Van de Velde M, Quesada AR, Medina MA, Noël A. García-Caballero M, et al. J Hematol Oncol. 2017 Jun 19;10(1):122. doi: 10.1186/s13045-017-0484-1. J Hematol Oncol. 2017. PMID: 28629427 Free PMC article. - Molecular control of lymphatic metastasis.
Achen MG, Stacker SA. Achen MG, et al. Ann N Y Acad Sci. 2008;1131:225-34. doi: 10.1196/annals.1413.020. Ann N Y Acad Sci. 2008. PMID: 18519975 Review. - Suppression of lymphangiogenesis in human lymphatic endothelial cells by simultaneously blocking VEGF-C and VEGF-D/VEGFR-3 with norcantharidin.
Liu ZY, Qiu HO, Yuan XJ, Ni YY, Sun JJ, Jing W, Fan YZ. Liu ZY, et al. Int J Oncol. 2012 Nov;41(5):1762-72. doi: 10.3892/ijo.2012.1603. Epub 2012 Aug 23. Int J Oncol. 2012. PMID: 22922710 - [Vascular endothelial growth factor (VEGF)-D in association with VEGF receptor-3 in lymphatic metastasis of breast cancer].
Chen YN, Gu Y. Chen YN, et al. Ai Zheng. 2009 Dec;28(12):1337-43. doi: 10.5732/cjc.009.10070. Ai Zheng. 2009. PMID: 19958632 Review. Chinese.
Cited by
- ZNF468-mediated epigenetic upregulation of VEGF-C facilitates lymphangiogenesis and lymphatic metastasis in ESCC via PI3K/Akt and ERK1/2 signaling pathways.
Zhu J, Qiu X, Jin X, Nie X, Ou S, Wu G, Shen J, Zhang R. Zhu J, et al. Cell Oncol (Dordr). 2024 Oct;47(5):1927-1942. doi: 10.1007/s13402-024-00976-0. Epub 2024 Aug 14. Cell Oncol (Dordr). 2024. PMID: 39141315 - Tumor-associated myeloid cells in cancer immunotherapy.
Cheng X, Wang H, Wang Z, Zhu B, Long H. Cheng X, et al. J Hematol Oncol. 2023 Jul 6;16(1):71. doi: 10.1186/s13045-023-01473-x. J Hematol Oncol. 2023. PMID: 37415162 Free PMC article. Review. - Enhancing immunotherapy response in melanoma: myeloid-derived suppressor cells as a therapeutic target.
Ozbay Kurt FG, Lasser S, Arkhypov I, Utikal J, Umansky V. Ozbay Kurt FG, et al. J Clin Invest. 2023 Jul 3;133(13):e170762. doi: 10.1172/JCI170762. J Clin Invest. 2023. PMID: 37395271 Free PMC article. Review. - A TMVP1-modified near-infrared nanoprobe: molecular imaging for tumor metastasis in sentinel lymph node and targeted enhanced photothermal therapy.
Wang X, Dai G, Jiang G, Zhang D, Wang L, Zhang W, Chen H, Cheng T, Zhou Y, Wei X, Li F, Ma D, Tan S, Wei R, Xi L. Wang X, et al. J Nanobiotechnology. 2023 Apr 17;21(1):130. doi: 10.1186/s12951-023-01883-6. J Nanobiotechnology. 2023. PMID: 37069646 Free PMC article. - Effect of crosstalk among conspirators in tumor microenvironment on niche metastasis of gastric cancer.
Zhu Z, Shi L, Dong Y, Zhang Y, Yang F, Wei J, Huo M, Li P, Liu X. Zhu Z, et al. Am J Cancer Res. 2022 Dec 15;12(12):5375-5402. eCollection 2022. Am J Cancer Res. 2022. PMID: 36628284 Free PMC article. Review.
References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Miscellaneous