Rab9-dependent retrograde transport and endosomal sorting of the endopeptidase furin - PubMed (original) (raw)
. 2011 Jul 15;124(Pt 14):2401-13.
doi: 10.1242/jcs.083782. Epub 2011 Jun 21.
Affiliations
- PMID: 21693586
- PMCID: PMC3124371
- DOI: 10.1242/jcs.083782
Rab9-dependent retrograde transport and endosomal sorting of the endopeptidase furin
Pei Zhi Cheryl Chia et al. J Cell Sci. 2011.
Abstract
The endopeptidase furin and the trans-Golgi network protein TGN38 are membrane proteins that recycle between the TGN and plasma membrane. TGN38 is transported by a retromer-dependent pathway from early endosomes to the TGN, whereas the intracellular transport of furin is poorly defined. Here we have identified the itinerary and transport requirements of furin. Using internalisation assays, we show that furin transits the early and late endosomes en route to the TGN. The GTPase Rab9 and the TGN golgin GCC185, components of the late endosome-to-TGN pathway, were required for efficient TGN retrieval of furin. By contrast, TGN38 trafficking was independent of Rab9 and GCC185. To identify the sorting signals for the early endosome-to-TGN pathway, the trafficking of furin-TGN38 chimeras was investigated. The diversion of furin from the Rab9-dependent late-endosome-to-TGN pathway to the retromer-dependent early-endosome-to-TGN pathway required both the transmembrane domain and cytoplasmic tail of TGN38. We present evidence to suggest that the length of the transmembrane domain is a contributing factor in endosomal sorting. Overall, these data show that furin uses the Rab9-dependent pathway from late endosomes and that retrograde transport directly from early endosomes is dependent on both the transmembrane domain and the cytoplasmic tail.
Figures
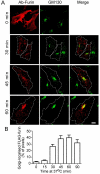
Fig. 1.
Trafficking of full-length furin from the plasma membrane to Golgi. (A) HeLa cells were transfected with FLAG–furin for 24 hours. Monolayers were incubated with anti-FLAG antibodies for 45 minutes on ice, washed in PBS, and either fixed (0 min) or incubated in serum-free medium for up to 90 minutes at 37°C to internalise the antibody–FLAG–furin complexes. Immediately following the incubation, monolayers were fixed and permeabilised and stained with Alexa-Fluor-568-conjugated anti-rabbit IgG (red). GM130 was stained with mouse monoclonal anti-GM130 antibodies, followed by Alexa-Fluor-488-conjugated anti-mouse IgG (green). Scale bar: 10 μm. (B) The number of FLAG–furin (red) pixels within the GM130-marked region was expressed as a percentage (±s.e.m.) of the total number of FLAG–furin pixels within each cell using the plug-in OBCOL on the ImageJ program (_n_=20 for each time point).
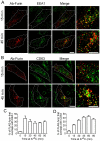
Fig. 2.
Furin is transported via the early and late endosomes en route to the TGN. HeLa cells were transfected with FLAG–furin for 24 hours and monolayers were incubated with anti-FLAG antibodies for 45 minutes on ice. Cells were washed in PBS and incubated in serum-free medium for 15 or 45 minutes at 37°C, then fixed, permeabilised and stained for internalised antibody–FLAG–furin complexes with Alexa-Fluor-568-conjugated anti-rabbit IgG (red) and for (A) EEA1 using mouse monoclonal anti-EEA1 antibodies (green) or (B) CD63 using mouse monoclonal anti-CD63 antibodies (green). Scale bars: 10 μm. The number of FLAG–furin pixels that overlapped with (C) EEA1 or (D) CD63 was expressed as a percentage (±s.e.m.) of the total number of FLAG–furin pixels within each cell using the plug-in OBCOL on the ImageJ program (_n_=20 for each time point).
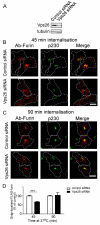
Fig. 3.
Retrograde transport of furin does not require Vps26. HeLa cells were transfected with either control siRNA or siRNA to knockdown Vps26 for 48 hours and transfected again with FLAG–furin for a further 24 hours. (A) For immunoblotting, cells were lysed in SDS-PAGE reducing buffer and cell extracts were subjected to SDS-PAGE on a 4–12% gradient polyacrylamide gel. Proteins were transferred to a PVDF membrane and probed with rabbit anti-Vps26 antibodies using a chemiluminescence detection system. The membrane was then stripped and reprobed with mouse anti-α-tubulin antibodies. (B,C) For internalisation assays, transfected cells were incubated with mouse anti-FLAG antibodies for 45 minutes on ice. Monolayers were washed in PBS and incubated in serum-free medium for (B) 45 minutes or (C) 90 minutes at 37°C and then fixed and permeabilised. These were stained for the internalised antibody–FLAG–furin complexes with Alexa-Fluor-568-conjugated anti-mouse IgG (red) and for p230 using rabbit anti-p230 antibodies, followed by Alexa-Fluor-488-conjugated anti-rabbit IgG (green). Scale bars: 10 μm. (D) Quantification of FLAG–furin levels within the Golgi of siRNA-treated cells. The percentage (±s.e.m.) of the total FLAG–furin pixels that overlapped with p230 in each cell was determined using the plug-in OBCOL on ImageJ (_n_=20 for each time-point). The data from Vps26-knockdown cells is expressed as a percentage of the control siRNA data set. ***P<0.001.
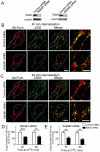
Fig. 4.
Retrograde transport of furin is dependent on Rab9. HeLa cells were transfected with either control siRNA, Rab9#1 siRNA or Rab9#2 siRNA, as indicated, for 48 hours and transfected again with FLAG–furin for a further 24 hours. (A) For immunoblotting, cells were lysed in SDS-PAGE reducing buffer and cell extracts were subjected to SDS-PAGE on a 4–12% gradient polyacrylamide gel. Proteins were transferred to a PVDF membrane and probed with mouse anti-Rab9 antibodies using a chemiluminescence detection system. The membrane was then stripped and reprobed with mouse anti-α-tubulin antibodies. For internalisation assays, transfected cells were incubated with rabbit anti-FLAG antibodies for 45 minutes on ice. Cells were washed in PBS and incubated in serum-free medium for 45 minutes or 90 minutes at 37°C and then fixed and permeabilised. These were stained for the internalised antibody-bound FLAG-furin with Alexa-Fluor-568-conjugated anti-rabbit IgG (red) and (B) p230 using human anti-p230 antibodies followed by Alexa-Fluor-647-conjugated anti-human IgG (pseudo-coloured green) or (C) CD63 using mouse monoclonal anti-CD63 antibodies, followed by Alexa-Fluor-488-conjugated anti-IgG (green). Scale bars: 10 μm. Quantification of FLAG-furin levels within the Golgi of cells treated with (D) Rab9#1 siRNA and (E) Rab9#2 siRNA. The percentage of the total FLAG–furin pixels that overlapped with p230 in each cell was determined using the plug-in OBCOL on ImageJ (_n_=20 for each time-point). The data from Rab9-knockdown cells is expressed as a percentage of the control siRNA data set (±s.e.m.). ***P<0.001; **P<0.01.
Fig. 5.
Furin requires the TGN golgin GCC185 for retrograde transport. HeLa cells were transfected with either control siRNA or siRNA against mRNA encoding GCC185 for 48 hours and transfected again with FLAG–furin for a further 24 hours. (A) Endogenous GCC185 was stained with affinity-purified rabbit anti-GCC185 antibodies, followed by Alexa-Fluor-568-conjugated anti-rabbit IgG. For immunoblotting, cells were lysed in SDS-PAGE reducing buffer and cell extracts were subjected to SDS-PAGE on a 4–12% gradient polyacrylamide gel. Proteins were transferred to a PVDF membrane and probed with affinity-purified rabbit anti-GCC185 antibodies using a chemiluminescence detection system. The membrane was then stripped and reprobed with mouse anti-α-tubulin antibodies. For internalisation assays, transfected cells were incubated with mouse monoclonal anti-FLAG antibodies for 45 minutes on ice. Cells were washed in PBS and incubated in serum-free medium for (B) 45 minutes or (C) 90 minutes at 37°C and then fixed and permeabilised. Monolayers were stained for the internalised antibody-bound FLAG–furin with Alexa-Fluor-568-conjugated anti-mouse IgG (red) and for p230 using rabbit anti-p230 antibodies, followed by Alexa-Fluor-488-conjugated anti-rabbit IgG (green). Scale bars: 10 μm. (D) Quantification of FLAG–furin levels within the Golgi of GCC185-knockdown cells. The percentage of the total FLAG–furin pixels that overlapped with p230 in each cell was determined using the plug-in OBCOL on ImageJ (_n_=20 for each time point). The data from GCC185-knockdown cells are expressed as a percentage (±s.e.m.) of the control siRNA data set. **P<0.01; ***P<0.001.
Fig. 6.
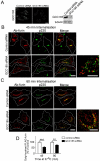
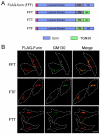
Steady-state distribution of chimeric constructs of furin and TGN38 expressed in HeLa cells. (A) Chimeric constructs of furin (blue) and TGN38 (green) were made by piecing together the lumenal domain of furin with the transmembrane domain of furin or TGN38 and the cytoplasmic tail of furin or TGN38. The FLAG epitope is indicated by the red segment. (B) HeLa cells were transfected with the FFT, FTF or FTT chimeric constructs for 24 hours and fixed and permeabilised. Monolayers were stained with rabbit polyclonal anti-FLAG antibodies (red) and mouse monoclonal anti-GM130 antibodies (green). Scale bar: 10 μm.
Fig. 7.
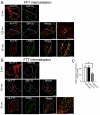
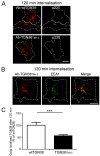
Intracellular trafficking of FFT and FTT. HeLa cells were transfected with FLAG-tagged (A) FFT or (B) FTT construct for 24 hours and incubated with anti-FLAG antibodies for 45 minutes on ice followed by washes with PBS. Monolayers were either fixed and permeabilised (0 min) or incubated in serum-free media for 30, 60 minutes or 120 minutes at 37°C and then fixed and permeabilised. Monolayers were stained for the internalised antibody-bound FLAG–FFT and FLAG–FTT with Alexa-Fluor-568-conjugated anti-rabbit IgG (red) and (A) for GM130 using mouse monoclonal anti-GM130 antibodies (green) or LAMP1 using mouse monoclonal anti-LAMP1 antibodies (green) and (B) for GM130 using mouse monoclonal anti-GM130 antibodies (green) or CD63 using mouse monoclonal anti-CD63 antibodies (green). (C) Quantification of FLAG–FFT and FLAG–FTT levels within the Golgi. The percentage (±s.e.m.) of the total FLAG pixels that overlapped with GM130 in each cell after 60 minutes internalisation was determined using the plug-in OBCOL on ImageJ (_n_=20 for each sample). The data from FLAG–FFT and FLAG–FTT is expressed as a percentage of the FLAG–furin data set. ***P<0.001. Scale bars: 10 μm.
Fig. 8.
Retrograde transport of FTT is Rab9 independent and Vps26 dependent. (A) HeLa cells were transfected with either control siRNA, Rab9#1 siRNA or siRNA to knockdown Vps26 for 48 hours and transfected again with FLAG–FTT for a further 24 hours. Monolayers were incubated with mouse anti-FLAG antibodies for 45 minutes on ice. Cells were washed in PBS and incubated in serum-free medium for 45 minutes at 37°C and then fixed and permeabilised. Monolayers were stained for the internalised antibody-bound FLAG–FTT with Alexa-Fluor-568-conjugated anti-mouse IgG (red) and for p230 with rabbit anti-p230 antibodies followed by Alexa-Fluor-488-conjugated anti-rabbit IgG (green). Scale bar: 10 μm. (B–D) Quantification of FLAG–FTT levels within the Golgi of siRNA-treated cells. The percentage of the total FLAG–FTT pixels that overlapped with p230 in each cell was determined using the plug-in OBCOL on ImageJ (_n_=20 for each sample). Data are expressed as a percentage (±s.e.m.) of the control siRNA data set. ***P<0.001.
Fig. 9.
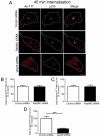
Increasing the length of the transmembrane domain of TGN38 reduces the efficiency of Golgi retrieval from early endosomes. HeLa cells were transfected with full-length TGN38 (wtTGN38) or TGN38TM+3 for 24 hours. Monolayers were incubated with mouse or rabbit anti-TGN38 antibodies for 30 minutes on ice, washed in PBS and incubated in serum-free medium for 120 minutes at 37°C and then fixed and permeabilised. (A,B) The internalised antibody-bound TGN38 or TGN38TM+3 was stained with (A) Alexa-Fluor-568-conjugated anti-mouse IgG (red) and p230 using rabbit polyclonal anti-p230 antibodies (green) or (B) Alexa-Fluor-568-conjugated anti-rabbit IgG (red) and EEA1 using mouse monoclonal anti-EEA1 (green). (C) Quantification of TGN38TM+3 levels at the Golgi. The percentage of the total TGN38 pixels that overlapped with p230 in each cell after 120 minutes of internalisation was determined using the plug-in OBCOL on ImageJ wtTGN38. Data are expressed as a percentage (±s.e.m.) of wtTGN38 (_n_=35 for each sample). ***P<0.001. Scale bars: 10 μm.
Fig. 10.
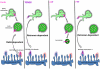
Model for endosomal sorting and retrograde transport of furin chimeras. Cartoon shows the distinct retrograde pathways of furin and TGN38, with furin transported by the Rab9-dependent late-endosome-to-TGN pathway and TGN38 depicted to segregate into the tubular structures of the early endosome for retromer-dependent transport to the TGN. The sorting model proposes that furin and FFT predominantly remain in the membrane of the body of the endosome, for subsequent trafficking to the late endosome, whereas FTT is segregated into tubular domains resulting in transport to TGN. Inclusion into the tubular domains is proposed to be dependent on both the transmembrane domain and cytoplasmic tail. The TGN golgins GCC88 and GCC185 are required for retrograde transport of TGN38 (Lieu et al., 2007) and furin (herein), respectively.
Similar articles
- Identification of different itineraries and retromer components for endosome-to-Golgi transport of TGN38 and Shiga toxin.
Lieu ZZ, Gleeson PA. Lieu ZZ, et al. Eur J Cell Biol. 2010 May;89(5):379-93. doi: 10.1016/j.ejcb.2009.10.021. Epub 2010 Feb 6. Eur J Cell Biol. 2010. PMID: 20138391 - The golgin GCC88 is required for efficient retrograde transport of cargo from the early endosomes to the trans-Golgi network.
Lieu ZZ, Derby MC, Teasdale RD, Hart C, Gunn P, Gleeson PA. Lieu ZZ, et al. Mol Biol Cell. 2007 Dec;18(12):4979-91. doi: 10.1091/mbc.e07-06-0622. Epub 2007 Oct 3. Mol Biol Cell. 2007. PMID: 17914056 Free PMC article. - Endosome to Golgi transport of ricin is independent of clathrin and of the Rab9- and Rab11-GTPases.
Iversen TG, Skretting G, Llorente A, Nicoziani P, van Deurs B, Sandvig K. Iversen TG, et al. Mol Biol Cell. 2001 Jul;12(7):2099-107. doi: 10.1091/mbc.12.7.2099. Mol Biol Cell. 2001. PMID: 11452006 Free PMC article. - From endosomes to the trans-Golgi network.
Lu L, Hong W. Lu L, et al. Semin Cell Dev Biol. 2014 Jul;31:30-9. doi: 10.1016/j.semcdb.2014.04.024. Epub 2014 Apr 23. Semin Cell Dev Biol. 2014. PMID: 24769370 Review. - Protein sorting from endosomes to the TGN.
Buser DP, Spang A. Buser DP, et al. Front Cell Dev Biol. 2023 Feb 21;11:1140605. doi: 10.3389/fcell.2023.1140605. eCollection 2023. Front Cell Dev Biol. 2023. PMID: 36895788 Free PMC article. Review.
Cited by
- The multiple roles of Rab9 in the endolysosomal system.
Kucera A, Bakke O, Progida C. Kucera A, et al. Commun Integr Biol. 2016 Jul 22;9(4):e1204498. doi: 10.1080/19420889.2016.1204498. eCollection 2016 Jul-Aug. Commun Integr Biol. 2016. PMID: 27574541 Free PMC article. - Delta opioid receptors recycle to the membrane after sorting to the degradation path.
Charfi I, Abdallah K, Gendron L, Pineyro G. Charfi I, et al. Cell Mol Life Sci. 2018 Jun;75(12):2257-2271. doi: 10.1007/s00018-017-2732-5. Epub 2017 Dec 29. Cell Mol Life Sci. 2018. PMID: 29288293 Free PMC article. - The Late Endosome and Its Lipid BMP Act as Gateways for Efficient Cytosolic Access of the Delivery Agent dfTAT and Its Macromolecular Cargos.
Erazo-Oliveras A, Najjar K, Truong D, Wang TY, Brock DJ, Prater AR, Pellois JP. Erazo-Oliveras A, et al. Cell Chem Biol. 2016 May 19;23(5):598-607. doi: 10.1016/j.chembiol.2016.03.016. Epub 2016 May 5. Cell Chem Biol. 2016. PMID: 27161484 Free PMC article. - Furin Egress from the TGN is Regulated by Membrane-Associated RING-CH Finger (MARCHF) Proteins and Ubiquitin-Specific Protease 32 (USP32) via Nondegradable K33-Polyubiquitination.
Su W, Ahmad I, Wu Y, Tang L, Khan I, Ye B, Liang J, Li S, Zheng YH. Su W, et al. Adv Sci (Weinh). 2024 Sep;11(35):e2403732. doi: 10.1002/advs.202403732. Epub 2024 Jul 19. Adv Sci (Weinh). 2024. PMID: 39031635 Free PMC article. - Mason-Pfizer Monkey Virus Envelope Glycoprotein Cycling and Its Vesicular Co-Transport with Immature Particles.
Prokšová PG, Lipov J, Zelenka J, Hunter E, Langerová H, Rumlová M, Ruml T. Prokšová PG, et al. Viruses. 2018 Oct 20;10(10):575. doi: 10.3390/v10100575. Viruses. 2018. PMID: 30347798 Free PMC article.
References
- Banting G., Ponnambalam S. (1997). TGN38 and its orthologues: roles in post-TGN vesicle formation and maintenance of TGN morphology. Biochim. Biophys. Acta 1355, 209-217 - PubMed
- Banting G., Maile R., Roquemore E. P. (1998). The steady state distribution of humTGN46 is not significantly altered in cells defective in clathrin-mediated endocytosis. J. Cell Sci. 111, 3451-3458 - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous