Asc-dependent and independent mechanisms contribute to restriction of legionella pneumophila infection in murine macrophages - PubMed (original) (raw)
doi: 10.3389/fmicb.2011.00018. eCollection 2011.
Mikhail A Gavrilin, Anwari Akhter, Kyle Caution, Sheetal Kotrange, Arwa Abu Khweek, Basant A Abdulrahman, Zeinab A Hassan, Fathia Z El-Sharkawi, Simranjit S Bedi, Katherine Ladner, M Elba Gonzalez-Mejia, Andrea I Doseff, Mahmoud Mostafa, Thirumala-Devi Kanneganti, Dennis Guttridge, Clay B Marsh, Mark D Wewers, Amal O Amer
Affiliations
- PMID: 21713115
- PMCID: PMC3112328
- DOI: 10.3389/fmicb.2011.00018
Asc-dependent and independent mechanisms contribute to restriction of legionella pneumophila infection in murine macrophages
Dalia H A Abdelaziz et al. Front Microbiol. 2011.
Abstract
The apoptosis-associated speck-like protein containing a caspase recruitment domain (Asc) is an adaptor molecule that mediates inflammatory and apoptotic signals. Legionella pneumophila is an intracellular bacterium and the causative agent of Legionnaire's pneumonia. L. pneumophila is able to cause pneumonia in immuno-compromised humans but not in most inbred mice. Murine macrophages that lack the ability to activate caspase-1, such as caspase(-1-/-) and Nlrc4(-/-) allow L. pneumophila infection. This permissiveness is attributed mainly to the lack of active caspase-1 and the absence of its down stream substrates such as caspase-7. However, the role of Asc in control of L. pneumophila infection in mice is unclear. Here we show that caspase-1 is moderately activated in Asc(-/-) macrophages and that this limited activation is required and sufficient to restrict L. pneumophila growth. Moreover, Asc-independent activation of caspase-1 requires bacterial flagellin and is mainly detected in cellular extracts but not in culture supernatants. We also demonstrate that the depletion of Asc from permissive macrophages enhances bacterial growth by promoting L. pneumophila-mediated activation of the NF-κB pathway and decreasing caspase-3 activation. Taken together, our data demonstrate that L. pneumophila infection in murine macrophages is controlled by several mechanisms: Asc-independent activation of caspase-1 and Asc-dependent regulation of NF-κB and caspase-3 activation.
Keywords: Asc; Legionella pneumophila; caspase-1; inflammasome.
Figures
Figure 1
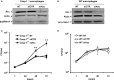
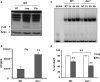
Asc controls Legionella pneumophila replication in caspase-1−/− macrophages. (A) Caspase-1−/− (casp-1−/−) macrophages and (B) WT macrophages were either left untreated (NT) or transfected with Asc specific siRNA (siAsc) or control siRNA (siCTR). After 48 h transfection Asc, levels were assessed using Western blotting. Actin was used as a loading control. Casp-1−/− macrophages (C) and wild type macrophages (D) were transfected or not (NT) with siAsc or siCTR and 48 h after transfection cells were infected with L. pneumophila (Leg) at MOI = 0.1 and the bacterial replication was assessed by counting the CFU after 1, 24, 48, and 72 h. Results are displayed as mean ± SD of three independent wells. **P ≤ 0.01. The data shown in (C,D) are representative of four independent experiments showing the same results.
Figure 2
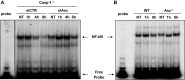
Asc hinders the activation of NF-κB induced by L. pneumophila. (A) Caspase-1−/− (casp-1−/−) macrophages were transfected with Asc specific siRNA (siAsc) or control siRNA (siCTR) and 48 h after transfection cells were infected or not (NT) with L. pneumophila (Leg) at MOI = 0.5 for 1, 4, and 8 h. Afterward, NF-κB activation was examined using electrophoretic mobility shift assay (EMSA). (B) WT and Asc−/− mouse macrophages were infected or not (NT) with L. pneumophila (Leg) at MOI = 0.5 for 1 and 8 h. Subsequently, NF-κB activation was examined using EMSA.
Figure 3
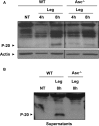
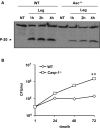
A fraction of caspase-1 is activated in the cytosol of murine macrophages lacking Asc. (A) WT and Asc−/− mouse macrophages were either not treated (NT) or infected with L. pneumophila (Leg) at MOI = 0.5 for 4 and 8 h. Then active caspase-1 (p-20) was detected in the cell extracts (A) and the supernatants (B) by Western blotting. Actin was used as a loading control.
Figure 4
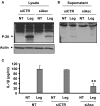
Depletion of Asc decreases caspase-1 activation in response to L. pneumophila infection. (A,B,C) WT mouse macrophages were transfected or not (NT) with Asc specific siRNA (siAsc) or control siRNA (siCtr) and 48 h after transfection cells were infected with L. pneumophila (Leg). Then, active caspase-1 (p-20) was detected in the cell extracts (A) and in the supernatants (B) of 8 h infected samples by Western blotting. Active IL-1β was detected in supernatants using ELISA (C). The results displayed as mean ± SD (**P ≤ 0.01) of three independent wells.
Figure 5
Asc−/− macrophages are permissive to flagellin mutant. (A) WT macrophages were infected with L. pneumophila (Leg), with corresponding flagellin mutant (Fla), or left untreated (NT). Active caspase-1 (p-20) was then detected in the cell extracts. (B) WT and Asc−/− macrophages were infected with Fla mutant and CFUs were scored 72 h after infection. (C) WT and Asc−/− macrophages were infected with Fla mutant for 1, 4, and 8 h then, nuclear extracts were processed for determination of NF-κB activation using electrophoretic mobility shift assay (EMSA). (D) WT and Asc macrophages were infected with Leg or Fla for 24 h then LDH release was determined and presented as percent cell death on the Y axis.
Figure 6
(A) Lysates from WT macrophages infected with L. pneumophila (Leg), the Fla mutant, or the type IV secretion mutant DotA were used to determine caspase-3 activity by the DEVD-AFC assay, or inactive full-length (FL-casp-3) and cleaved caspase-3 (cleaved casp-3) by immunoblotting. (B) WT and Asc−/− macrophages were infected with Fla mutant for 1, 2, and 5 h then cell lysates were obtained and used to determine cleaved caspase-3 by Western Blotting.
Figure A1
(A) Wild type (WT) mouse macrophages and heterozygous Asc (Asc−/+) were infected or not (NT) with L. pneumophila (Leg) at MOI = 0.5 for 1, 2, and 4 h. Total active caspase-1 (p-20) was detected in combined lysate and supernatants by Western blotting. (B) WT and caspase-1−/− macrophages were infected with L. pneumophila (Leg) at MOI = 0.1 and the bacterial replication was assessed by counting the colony-forming units (CFU) after 1, 24, 48, and 72 h. Results are displayed as mean ± SD of three independent wells. **P ≤ 0.01.
Figure A2
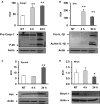
Legionella pneumophila differentially regulates the expression of several components of the inflammasome. WT mouse macrophages were infected or not (NT) with Legionella pneumophila (Leg) at MOI = 0.5 for 4 and 24 h. then the expressions of (A) pro-caspase-1, (B) pro-Il-1β, (C) Pycard (Asc), and (D) Nlrc4 (Ipaf) were then assessed on both mRNA (upper panels) and protein levels (lower panels) using RT-PCR and Western blots, respectively. The data of RT-PCR are displayed as mean relative copy numbers (RCN) ± SD of three independent experiments. *P ≤ 0.05, **P ≤ 0.01. Actin was used as loading control.
Similar articles
- Asc modulates the function of NLRC4 in response to infection of macrophages by Legionella pneumophila.
Case CL, Roy CR. Case CL, et al. mBio. 2011 Sep 1;2(4):e00117-11. doi: 10.1128/mBio.00117-11. Print 2011. mBio. 2011. PMID: 21771913 Free PMC article. - Asc and Ipaf Inflammasomes direct distinct pathways for caspase-1 activation in response to Legionella pneumophila.
Case CL, Shin S, Roy CR. Case CL, et al. Infect Immun. 2009 May;77(5):1981-91. doi: 10.1128/IAI.01382-08. Epub 2009 Feb 23. Infect Immun. 2009. PMID: 19237518 Free PMC article. - Caspase-7 activation by the Nlrc4/Ipaf inflammasome restricts Legionella pneumophila infection.
Akhter A, Gavrilin MA, Frantz L, Washington S, Ditty C, Limoli D, Day C, Sarkar A, Newland C, Butchar J, Marsh CB, Wewers MD, Tridandapani S, Kanneganti TD, Amer AO. Akhter A, et al. PLoS Pathog. 2009 Apr;5(4):e1000361. doi: 10.1371/journal.ppat.1000361. Epub 2009 Apr 3. PLoS Pathog. 2009. PMID: 19343209 Free PMC article. - Modulation of caspases and their non-apoptotic functions by Legionella pneumophila.
Amer AO. Amer AO. Cell Microbiol. 2010 Feb;12(2):140-7. doi: 10.1111/j.1462-5822.2009.01401.x. Epub 2009 Oct 27. Cell Microbiol. 2010. PMID: 19863553 Review. - Caspase Exploitation by Legionella pneumophila.
Krause K, Amer AO. Krause K, et al. Front Microbiol. 2016 Apr 13;7:515. doi: 10.3389/fmicb.2016.00515. eCollection 2016. Front Microbiol. 2016. PMID: 27148204 Free PMC article. Review.
Cited by
- Cellular microbiology and molecular ecology of Legionella-amoeba interaction.
Richards AM, Von Dwingelo JE, Price CT, Abu Kwaik Y. Richards AM, et al. Virulence. 2013 May 15;4(4):307-14. doi: 10.4161/viru.24290. Epub 2013 Mar 27. Virulence. 2013. PMID: 23535283 Free PMC article. Review. - A New Twist in the Regulation of Legionella Replication through ASC and Caspase-1.
Franchi L, Núñez G. Franchi L, et al. Front Microbiol. 2011 Mar 21;2:57. doi: 10.3389/fmicb.2011.00057. eCollection 2011. Front Microbiol. 2011. PMID: 21833313 Free PMC article. No abstract available. - Shiga Toxins Activate the NLRP3 Inflammasome Pathway To Promote Both Production of the Proinflammatory Cytokine Interleukin-1β and Apoptotic Cell Death.
Lee MS, Kwon H, Lee EY, Kim DJ, Park JH, Tesh VL, Oh TK, Kim MH. Lee MS, et al. Infect Immun. 2015 Oct 26;84(1):172-86. doi: 10.1128/IAI.01095-15. Print 2016 Jan. Infect Immun. 2015. PMID: 26502906 Free PMC article. - Caspase-11 promotes the fusion of phagosomes harboring pathogenic bacteria with lysosomes by modulating actin polymerization.
Akhter A, Caution K, Abu Khweek A, Tazi M, Abdulrahman BA, Abdelaziz DH, Voss OH, Doseff AI, Hassan H, Azad AK, Schlesinger LS, Wewers MD, Gavrilin MA, Amer AO. Akhter A, et al. Immunity. 2012 Jul 27;37(1):35-47. doi: 10.1016/j.immuni.2012.05.001. Epub 2012 May 31. Immunity. 2012. PMID: 22658523 Free PMC article. - Receptor interacting protein-2 plays a critical role in human lung epithelial cells survival in response to Fas-induced cell-death.
Rahman MA, Sundaram K, Mitra S, Gavrilin MA, Wewers MD. Rahman MA, et al. PLoS One. 2014 Mar 21;9(3):e92731. doi: 10.1371/journal.pone.0092731. eCollection 2014. PLoS One. 2014. PMID: 24658576 Free PMC article.
References
- Akhter A., Gavrilin M. A., Frantz L., Washington S., Ditty C., Limoli D., Day C., Sarkar A., Newland C., Butchar J., Marsh C. B., Wewers M. D., Tridandapani S., Kanneganti T. D., Amer A. O. (2009). Caspase-7 activation by the Nlrc4/Ipaf inflammasome restricts Legionella pneumophila infection. PLoS Pathog. 5, e1000361.10.1371/journal.ppat.1000361 - DOI - PMC - PubMed
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous