MYCN sensitizes neuroblastoma to the MDM2-p53 antagonists Nutlin-3 and MI-63 - PubMed (original) (raw)
MYCN sensitizes neuroblastoma to the MDM2-p53 antagonists Nutlin-3 and MI-63
L D Gamble et al. Oncogene. 2012.
Abstract
MYCN amplification is a major biomarker of poor prognosis, occurring in 25-30% of neuroblastomas. MYCN has contradictory roles in promoting cell growth and sensitizing cells to apoptosis. We have recently shown that p53 is a direct transcriptional target of MYCN in neuroblastoma and that p53-mediated apoptosis may be an important mechanism of MYCN-induced apoptosis. Although p53 mutations are rare in neuroblastoma at diagnosis, the p53/MDM2/p14(ARF) pathway is often inactivated through MDM2 amplification or p14(ARF) inactivation. We hypothesized that reactivation of p53 by inhibition of its negative regulator MDM2, using the MDM2-p53 antagonists Nutlin-3 and MI-63, will result in p53-mediated growth arrest and apoptosis especially in MYCN-amplified cells. Using the SHEP Tet21N MYCN-regulatable system, MYCN(-) cells were more resistant to both Nutlin-3 and MI-63 mediated growth inhibition and apoptosis compared with MYCN(+) cells and siRNA-mediated knockdown of MYCN in four MYCN-amplified cell lines resulted in decreased p53 expression and activation, as well as decreased levels of apoptosis following treatment with MDM2-p53 antagonists. In a panel of 18 neuroblastoma cell lines treated with Nutlin-3 and MI-63, the subset amplified for MYCN had a significantly lower mean GI(50) value (50% growth inhibition) and increased caspase 3/7 activity compared with the non-MYCN-amplified group of cell lines, but p53 mutant cell lines were resistant to the antagonists regardless of MYCN status. We conclude that amplification or overexpression of MYCN sensitizes neuroblastoma cell lines with wild-type p53 to MDM2-p53 antagonists and that these compounds may therefore be particularly effective in treating high-risk MYCN-amplified disease.
Figures
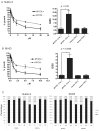
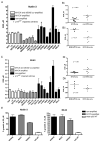
Figure 1. MYCN(+) Tet21N cells are more sensitive to MDM2-p53 antagonist mediated growth inhibition than MYCN(−) Tet21N cells
Growth inhibition assays for A) Nutlin-3 and B) MI-63 were performed after 72 hour drug exposure. MYCN(+) cells have a significantly lower mean GI50 compared to MYCN(−) cells (p=0.02 for Nutlin-3 and p=0.0008 for MI-63, paired t-test). No difference in GI50s was observed between the control Tet21 cells in the presence and absence of tetracycline. C) Cell cycle analysis after 24 hour drug exposure shows that both MYCN(−) and MYCN(+) cells G1 arrest in response to 2.5μM and 10μM Nutlin-3 or MI-63, and show that MYCN(−) cells have an increased proportion of cells in G1 in control samples compared to MYCN(+) cells.
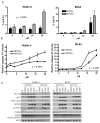
Figure 2. MYCN(+) Tet21N cells are more sensitive to MDM2-p53 antagonist mediated apoptosis than MYCN(−) Tet21N cells, following 24 hours treatment
A) The sub G1 DNA fraction was significantly lower in MYCN(−) compared to MYCN(+) cells following Nutlin-3 treatment (p=0.0007, 2-way ANOVA) and although of borderline significance, a decrease in the sub G1 fraction is observed in MYCN(−) cells compared to MYCN(+) cells following MI-63 treatment. (p=0.058 for MI-63, 2-Way ANOVA), B) Caspase 3/7 activity was significantly reduced in MYCN(−) compared to MYCN(+) cells following Nutlin-3 and MI-63 treatment (p<0.0001 for both Nutlin-3, and MI-63, 2-way ANOVA). C) Western blots showed no difference in induction of p53, MDM2 and p21WAF1 in MYCN(−) and MYCN(+) cells following Nutlin-3 and MI-63 treatment but there were increased levels of phosphorylated p53 and PUMA in MYCN(+) compared to MYCN(−) cells. Actin was used as a loading control.
Figure 3. siRNA knockdown of MYCN (30nmol/L for 24 hours) decreases the sensitivity of MYCN and MDM2 co-amplified TR14 cells to Nutlin-3 and MI-63 treatment (24 hours)
A) Western blot showing induction of p53 responsive genes and levels of apoptotic markers. Actin was used as a loading control. B) Caspase 3/7 activity was significantly reduced compared to SCR siRNA following MYCN knockdown (p=0.0015 for Nutlin-3, p=0.0001 for MI-63, 2-way ANOVA). C) Cell cycle analysis showed a slight G1 arrest following Nutlin-3 or MI-63 treatment and no further effect upon MYCN knockdown despite MYCN knockdown alone inducing a slight G1 arrest.
Figure 4. siRNA knockdown of MYCN (50nmol/L for 24 hours) decreases the sensitivity of MYCN amplified LAN5 cells to MDM2-p53 antagonist treatment _(_24 hours)
A) Western blot showing the effect on the p53 response and levels of apoptotic markers. Actin was used as a loading control. B) Caspase 3/7 activity was significantly reduced following MYCN knockdown (p<0.0026 for Nutlin-3, p<0.008 for MI-63, 2-way ANOVA). C) Cell cycle analysis showed no G1 arrest following Nutlin-3 or MI-63 treatment and no further effect upon MYCN knockdown.
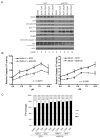
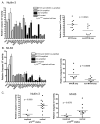
Figure 5. MYCN amplified neuroblastoma cell lines are more sensitive to MDM2-p53 antagonists than non-MYCN amplified neuroblastoma cell lines
A panel of neuroblastoma cell lines were treated with A), B) Nutlin-3 and C), D) MI-63 with doses ranging from 0-20μM and 0-10μM respectively to obtain GI50s. A significant difference was observed between GI50s for both Bi) Nutlin-3 (p<0.001) and Di) MI-63 (p<0.05). There was an increase in GI50s in MYCN and MDM2 co-amplified cell lines compared to MYCN only amplified cell lines for Bii) Nutlin-3 (p<0.01) but not Dii) MI-63 (p=0.07). Mann-Whitney tests were used to calculate p values. E) p53 mutant cell lines including one non-amplified and 2 MYCN amplified were treated with up to 20μM Nutlin-3 and 10μM MI-63. All three cell lines were resistant to Nutlin-3 and MI-63 compared to wildtype p53 cell lines. Cell lines marked * are p14ARF impaired (methylation or homozygous deletion).
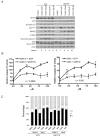
Figure 6. MYCN amplified neuroblastoma cell lines are more sensitive to MDM2-p53 antagonists mediated apoptosis than non-MYCN amplified neuroblastoma cell lines
A panel of neuroblastoma cell lines were treated with A) 5μM Nutlin-3 and B) 2.5μM MI-63 and relative caspase 3/7 activity determined. The difference in caspase 3/7 activity was significantly higher in MYCN amplified compared to non-MYCN amplified cell lines (p=0.0343 for Nutlin-3, and p=0.0085 for MI-63). p53 mutant cell lines had low levels of caspase activity. Cell lines marked * are p14ARF impaired (methylation or homozygous deletion). C) p14ARF impaired cell lines have significantly lower levels of caspase 3/7 activity compared to cell lines with normal p14ARF (p=0.0093 for Nutlin-3, p=0.0078 for MI-63, Mann-Whitney test).
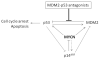
Figure 7. MYCN is a central modulator of the p53/MDM2/p14ARF negative feedback loop
MDM2-p53 antagonists block the MDM2-p53 interaction.
Similar articles
- Characterisation of the p53 pathway in cell lines established from TH-MYCN transgenic mouse tumours.
Chen L, Esfandiari A, Reaves W, Vu A, Hogarty MD, Lunec J, Tweddle DA. Chen L, et al. Int J Oncol. 2018 Mar;52(3):967-977. doi: 10.3892/ijo.2018.4261. Epub 2018 Jan 31. Int J Oncol. 2018. PMID: 29393340 - Galectin-3 impairment of MYCN-dependent apoptosis-sensitive phenotype is antagonized by nutlin-3 in neuroblastoma cells.
Veschi V, Petroni M, Cardinali B, Dominici C, Screpanti I, Frati L, Bartolazzi A, Gulino A, Giannini G. Veschi V, et al. PLoS One. 2012;7(11):e49139. doi: 10.1371/journal.pone.0049139. Epub 2012 Nov 9. PLoS One. 2012. PMID: 23152863 Free PMC article. - Crosstalk between MYCN and MDM2-p53 signal pathways regulates tumor cell growth and apoptosis in neuroblastoma.
He J, Gu L, Zhang H, Zhou M. He J, et al. Cell Cycle. 2011 Sep 1;10(17):2994-3002. doi: 10.4161/cc.10.17.17118. Epub 2011 Sep 1. Cell Cycle. 2011. PMID: 21862876 Free PMC article. - MDM2 as MYCN transcriptional target: implications for neuroblastoma pathogenesis.
Slack A, Lozano G, Shohet JM. Slack A, et al. Cancer Lett. 2005 Oct 18;228(1-2):21-7. doi: 10.1016/j.canlet.2005.01.050. Cancer Lett. 2005. PMID: 15927364 Review. - Targeting the p53-MDM2 pathway for neuroblastoma therapy: Rays of hope.
Zafar A, Wang W, Liu G, Xian W, McKeon F, Zhou J, Zhang R. Zafar A, et al. Cancer Lett. 2021 Jan 1;496:16-29. doi: 10.1016/j.canlet.2020.09.023. Epub 2020 Sep 29. Cancer Lett. 2021. PMID: 33007410 Free PMC article. Review.
Cited by
- MYCN Amplification, along with Wild-Type RB1 Expression, Enhances CDK4/6 Inhibitors' Efficacy in Neuroblastoma Cells.
De Rosa P, Severi F, Zadran SK, Russo M, Aloisi S, Rigamonti A, Capranico G, Milazzo G, Perini G. De Rosa P, et al. Int J Mol Sci. 2023 Mar 12;24(6):5408. doi: 10.3390/ijms24065408. Int J Mol Sci. 2023. PMID: 36982482 Free PMC article. - Deciphering the Role of p53 and TAp73 in Neuroblastoma: From Pathogenesis to Treatment.
Almeida J, Mota I, Skoda J, Sousa E, Cidade H, Saraiva L. Almeida J, et al. Cancers (Basel). 2022 Dec 16;14(24):6212. doi: 10.3390/cancers14246212. Cancers (Basel). 2022. PMID: 36551697 Free PMC article. Review. - LIN28B inhibition sensitizes cells to p53-restoring PPI therapy through unleashed translational suppression.
Shi J, Jin X, Wang Y, Zhu T, Zhang D, Li Q, Zhong X, Deng Y, Shen J, Fan X. Shi J, et al. Oncogenesis. 2022 Jul 2;11(1):37. doi: 10.1038/s41389-022-00412-8. Oncogenesis. 2022. PMID: 35780125 Free PMC article. - Dodging the bullet: therapeutic resistance mechanisms in pediatric cancers.
Shah N. Shah N. Cancer Drug Resist. 2019 Sep 19;2(3):428-446. doi: 10.20517/cdr.2019.20. eCollection 2019. Cancer Drug Resist. 2019. PMID: 35582583 Free PMC article. Review. - p53-Mediated Radiosensitization of 177Lu-DOTATATE in Neuroblastoma Tumor Spheroids.
Lundsten S, Berglund H, Jha P, Krona C, Hariri M, Nelander S, Lane DP, Nestor M. Lundsten S, et al. Biomolecules. 2021 Nov 15;11(11):1695. doi: 10.3390/biom11111695. Biomolecules. 2021. PMID: 34827693 Free PMC article.
References
- Amente S, Gargano B, Diolaiti D, Della Valle G, Lania L, Majello B. p14ARF interacts with N-Myc and inhibits its transcriptional activity. FEBS Lett. 2007;581:821–825. - PubMed
- Barbieri E, Mehta P, Chen Z, Zhang L, Slack A, Berg S, et al. MDM2 inhibition sensitizes neuroblastoma to chemotherapy-induced apoptotic cell death. Mol Cancer Ther. 2006;5:2358–2365. - PubMed
- Bell E, Premkumar R, Carr J, Lu X, Lovat PE, Kees UR, et al. The role of MYCN in the failure of MYCN amplified neuroblastoma cell lines to G1 arrest after DNA damage. Cell Cycle. 2006;5:2639–2647. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Research Materials
Miscellaneous