Repeated cleavage failure does not establish centrosome amplification in untransformed human cells - PubMed (original) (raw)
Repeated cleavage failure does not establish centrosome amplification in untransformed human cells
Anna Krzywicka-Racka et al. J Cell Biol. 2011.
Abstract
We tested whether cleavage failure as a transient event establishes an incidence of centrosome amplification in cell populations. Five rounds of ∼30% cytochalasin-induced cleavage failure in untransformed human cell cultures did not establish centrosome amplification in the short or long terms. The progeny of binucleate cells progressively dropped out of the cell cycle and expressed p53/p21, and none divided a fourth time. We also tested whether cleavage failure established centrosome amplification in transformed cell populations. Tetraploid HCT116 p53(-/-) cells eventually all failed cleavage repeatedly and ceased proliferating. HeLa cells all died or arrested within four cell cycles. Chinese hamster ovary cells proliferated after cleavage failure, but five rounds of induced cleavage failure produced a modest increase in the incidence of centrosome amplification in the short term, which did not rise with more cycles of cleavage failure. This incidence dropped to close to control values in the long term despite a 2-6% rate of spontaneous cleavage failure in the progeny of tetraploid cells.
Figures
Figure 1.
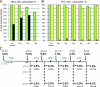
Centrosome amplification and proliferation of untransformed cells after cleavage failure. (A) Centrosome amplification after repeated cleavage failure in RPE1 cell populations. Asynchronous cultures were treated with 0.5 µM cytochalasin D for 10 h (green bars) and split 100 h later. One culture was passaged seven times; the other was treated again with cytochalasin. This was repeated four more times. The percentages of binuclear cells after cytochalasin treatments are shown in blue. The percentages of centrosome amplification (more than four centrioles) are shown in bold with the number of cells counted shown above or below. (B) Proliferative capacity of binuclear cells and their progeny (yellow bars) and same-preparation control cells (green bars) after cytochalasin-induced cleavage failure. Bars show the percentage of cells that enter mitosis after completing the prior mitosis for each cell cycle; cells arresting in a previous cell cycle are not included. Percentages were calculated from lineages of multiple individual cells followed in one experiment. (C) Proliferative capacity of binuclear cells (yellow bars) and same-preparation control cells (green bars) after blebbistatin-induced cleavage failure. One experiment is shown on multiple cells.
Figure 2.
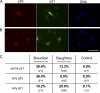
Expression of p53 and p21 in untransformed cells that stop cycling after cleavage failure. (A) Binuclear cell arrested after cleavage failure expressing p53 (red), p21 (green), and DNA (blue). (B) Daughters of a binuclear cell fixed 15 h after first tetraploid mitosis showing p53 (red) and p21 (green). (C) Percentages of cells expressing only p53, only p21, or both. The first column shows binucleates that arrested in first interphase, the second column shows daughters of binucleates, and the third column shows same-preparation control cells. Fluorescence micrographs are shown. Bar, 50 µm.
Figure 3.
Centrosome amplification and proliferation of HCT116 p53−/− cells after cleavage failure. (A) Centrosome amplification in proliferative HCT116 p53−/− cell populations after repeated cleavage failure. The experimental protocol and display of the results are the same as those shown in Fig. 1 A. Large multinucleated cells are not scored because they do not proliferate. The percentages of binuclear cells after cytochalasin treatments are shown in blue. The percentages of centrosome amplification (more than four centrioles) are shown in bold with the number of cells counted shown above or below. (B) Proliferative capacity of binuclear cells (yellow bars) and same-preparation control cells (green bars). Orange portions of the bars indicate the percentages of cells that spontaneously fail cleavage at mitosis. Such cells cycle but repeatedly fail cleavage and cease proliferating. One experiment is shown on multiple cells. (C, top) Binuclear cell (asterisks) exhibiting multiple rounds of cleavage failure, resulting in a large multinucleated cell. (bottom) Death of another large multinuclear cell. Times are in hours and minutes. Phase-contrast microscopy is shown. Bar, 50 µm.
Figure 4.
Centrosome amplification and proliferation of HeLa and CHO cells after cleavage failure. (A) Proliferative capacity of binuclear HeLa cells (yellow bars) and same preparation control cells (green bars). Black portions of bars indicate the percentage of the cells that die during or just after that mitosis. (B) Proliferative capacity of binuclear CHO cells (yellow bars) and same-preparation control cells (green bars). The darker portions of the yellow bars denote the percentage of cells that fail cleavage at each mitosis. (A and B) One experiment is shown on multiple cells. (C) Centrosome amplification after repeated cleavage failure in CHO cell populations. The experimental protocol and display of the results are the same as those shown in Fig. 1 A. The percentages of binuclear cells after cytochalasin treatments are shown in blue. The percentages of centrosome amplification (more than four centrioles) are shown in bold with the number of cells counted shown above or below.
Similar articles
- Multiple centrosomes arise from tetraploidy checkpoint failure and mitotic centrosome clusters in p53 and RB pocket protein-compromised cells.
Borel F, Lohez OD, Lacroix FB, Margolis RL. Borel F, et al. Proc Natl Acad Sci U S A. 2002 Jul 23;99(15):9819-24. doi: 10.1073/pnas.152205299. Epub 2002 Jul 15. Proc Natl Acad Sci U S A. 2002. PMID: 12119403 Free PMC article. - [The p53-p21(waf1) pathway and centrosome amplification in oral squamous cell carcinomas].
Cai Y, Liu YF, Yang H, Lu H. Cai Y, et al. Zhonghua Kou Qiang Yi Xue Za Zhi. 2009 Jun;44(6):332-5. Zhonghua Kou Qiang Yi Xue Za Zhi. 2009. PMID: 19953948 Chinese. - Dissociation of centrosome replication events from cycles of DNA synthesis and mitotic division in hydroxyurea-arrested Chinese hamster ovary cells.
Balczon R, Bao L, Zimmer WE, Brown K, Zinkowski RP, Brinkley BR. Balczon R, et al. J Cell Biol. 1995 Jul;130(1):105-15. doi: 10.1083/jcb.130.1.105. J Cell Biol. 1995. PMID: 7790366 Free PMC article. - Deregulation of the centrosome cycle and the origin of chromosomal instability in cancer.
Lingle WL, Lukasiewicz K, Salisbury JL. Lingle WL, et al. Adv Exp Med Biol. 2005;570:393-421. doi: 10.1007/1-4020-3764-3_14. Adv Exp Med Biol. 2005. PMID: 18727509 Review. - The interplay between centrosomes and the Hippo tumor suppressor pathway.
Bolgioni AF, Ganem NJ. Bolgioni AF, et al. Chromosome Res. 2016 Jan;24(1):93-104. doi: 10.1007/s10577-015-9502-8. Chromosome Res. 2016. PMID: 26582635 Free PMC article. Review.
Cited by
- Oxidative Stress in Cells with Extra Centrosomes Drives Non-Cell-Autonomous Invasion.
Arnandis T, Monteiro P, Adams SD, Bridgeman VL, Rajeeve V, Gadaleta E, Marzec J, Chelala C, Malanchi I, Cutillas PR, Godinho SA. Arnandis T, et al. Dev Cell. 2018 Nov 19;47(4):409-424.e9. doi: 10.1016/j.devcel.2018.10.026. Dev Cell. 2018. PMID: 30458137 Free PMC article. - Keep Calm and Carry on with Extra Centrosomes.
Kalkan BM, Ozcan SC, Quintyne NJ, Reed SL, Acilan C. Kalkan BM, et al. Cancers (Basel). 2022 Jan 17;14(2):442. doi: 10.3390/cancers14020442. Cancers (Basel). 2022. PMID: 35053604 Free PMC article. Review. - Permission to pass: on the role of p53 as a gatekeeper for aneuploidy.
Marques JF, Kops GJPL. Marques JF, et al. Chromosome Res. 2023 Oct 21;31(4):31. doi: 10.1007/s10577-023-09741-9. Chromosome Res. 2023. PMID: 37864038 Free PMC article. Review. - Centrosomes, chromosome instability (CIN) and aneuploidy.
Vitre BD, Cleveland DW. Vitre BD, et al. Curr Opin Cell Biol. 2012 Dec;24(6):809-15. doi: 10.1016/j.ceb.2012.10.006. Epub 2012 Nov 3. Curr Opin Cell Biol. 2012. PMID: 23127609 Free PMC article. Review. - Centrosome dysfunction contributes to chromosome instability, chromoanagenesis, and genome reprograming in cancer.
Pihan GA. Pihan GA. Front Oncol. 2013 Nov 12;3:277. doi: 10.3389/fonc.2013.00277. eCollection 2013. Front Oncol. 2013. PMID: 24282781 Free PMC article. Review.
References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous